NCERT Solutions Class 11 Chemistry Chapter 3 - Classification of Elements & Periodicity in Properties
| Table of contents |

|
| NCERT Questions on Page No. 96 |

|
| NCERT Questions on Page No. 97 |

|
| NCERT Questions on Page No. 98 |

|
| NCERT Questions on Page No. 99 |

|
NCERT Questions on Page No. 96
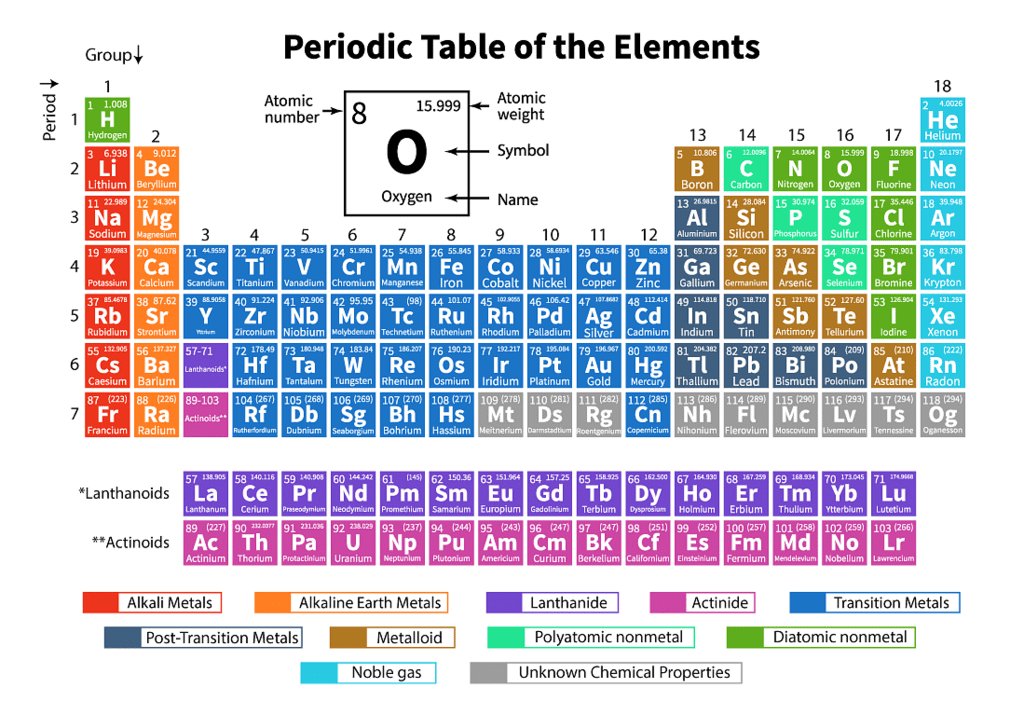
Q3.1: What is the basic theme of organization in the periodic table?
Ans: The basic theme of organisation of elements in the periodic table is to classify the elements in periods and groups according to their properties. This arrangement makes the study of elements and their compounds simple and systematic. In the periodic table, elements with similar properties are placed in the same group.
Q3.2: Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
Ans: Mendeleev arranged the elements in his periodic table in order of increasing atomic weight (atomic mass). He placed the elements in periods and groups, such that elements with similar properties were in the same group.
However, he did not stick to this arrangement strictly. He found that if the elements were arranged only by atomic weights, some did not fit well.
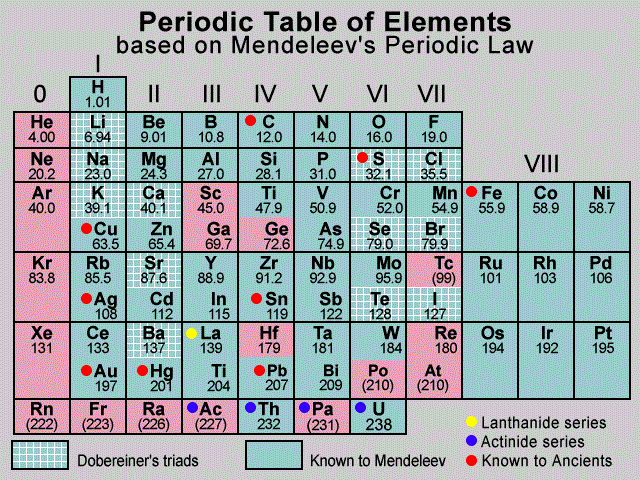 Mendeleev's Periodic Table
Mendeleev's Periodic Table
Example: The atomic weight of iodine is less than that of tellurium. Still, Mendeleev placed tellurium (Group VI) before iodine (Group VII), since iodine’s properties were closer to fluorine, chlorine, and bromine.
Q3.3: What is the basic difference in approach between the Mendeleev’s Periodic Law and the Modern Periodic Law?
Ans:
Mendeleev’s Periodic Law: The physical and chemical properties of elements are periodic functions of their atomic weights.
Modern Periodic Law: The physical and chemical properties of elements are periodic functions of their atomic numbers.
Q3.4: On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
Ans: In the periodic table, a period corresponds to the principal quantum number (n) of the outermost shell.
- For the sixth period, n = 6.
- For n = 6, the azimuthal quantum number (l) can have values 0, 1, 2, 3, 4.
- Subshells involved: 6s, 4f, 5d, and 6p.
Orbitals available:
- 6s → 1 orbital
- 4f → 7 orbitals
- 5d → 5 orbitals
- 6p → 3 orbitals
Total = 1 + 7 + 5 + 3 = 16 orbitals.
Since each orbital can accommodate 2 electrons (Pauli’s principle), total capacity = 16 × 2 = 32 electrons.
Hence, the sixth period contains 32 elements.
Q3.5: In terms of period and group where would you locate the element with Z =114?
Ans: Elements with atomic numbers Z = 87 to 114 lie in the 7th period.
- Z = 87, 88 → s-block elements
- Z = 89, 104–112 → d-block elements
- Z = 90–103 → f-block elements
- Z = 113–118 → p-block elements
Thus, the element with Z = 114 is a p-block element in the 7th period.
It lies in Group 14 of the periodic table.
Q3.6: Write the atomic number of the element present in the third period and seventeenth group of the periodic table.
Ans: The element is chlorine (Cl) with atomic number (Z) = 17.
Q3.7: Which element do you think would have been named by
(i) Lawrence Berkeley Laboratory
(ii) Seaborg’s group?
Ans:
(i) Lawrencium (Lr) with Z = 103 and Berkelium (Bk) with Z = 97
(ii) Seaborgium (Sg) with Z = 106
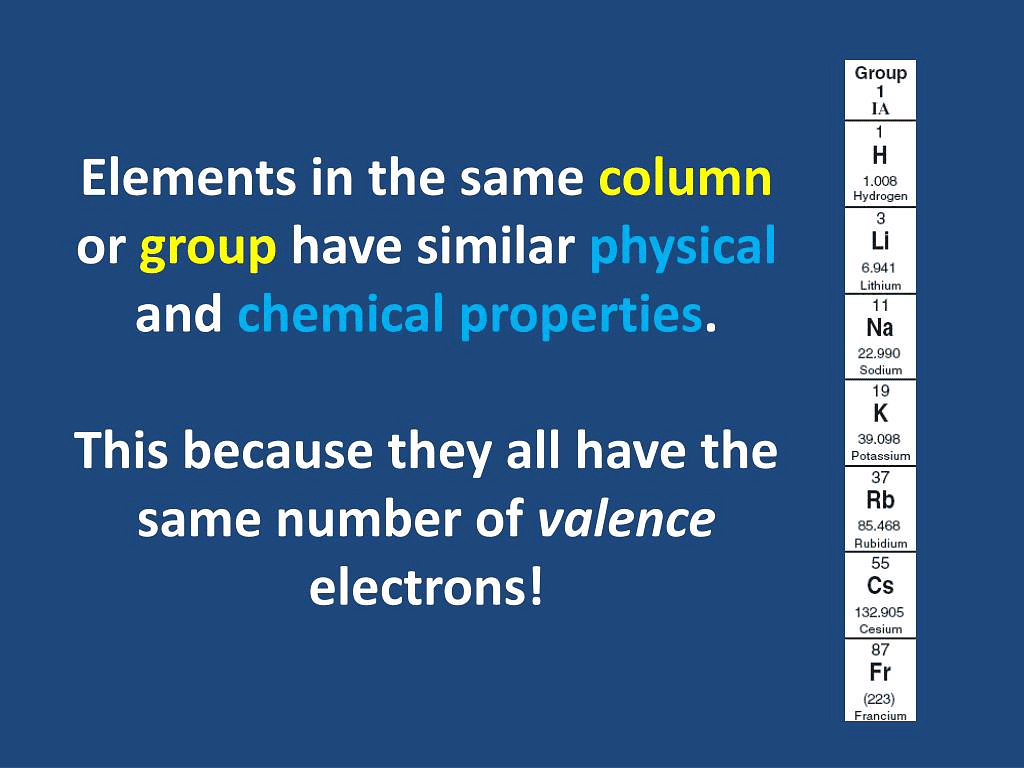
Q3.8: Why do elements in the same group have similar physical and chemical properties?
Ans: The physical and chemical properties of elements depend on the number of valence electrons. Elements present in the same group have the same number of valence electrons. Therefore, elements present in the same group have similar physical and chemical properties.
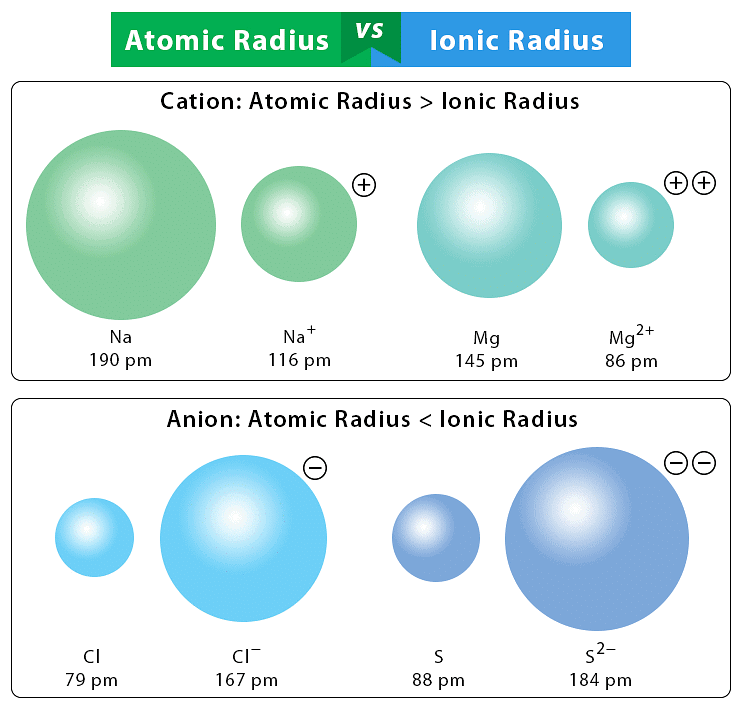
Q3.9: What does atomic radius and ionic radius really mean to you?
Ans: Atomic radius: The distance from the nucleus to the outermost shell of an atom.
- Metallic radius: Half the distance between two adjacent atoms in a metallic crystal.
Example: Cu–Cu distance = 256 pm ⇒ metallic radius = 128 pm. - Covalent radius: Half the distance between two atoms joined by a covalent bond.
Example: Cl–Cl bond length = 198 pm ⇒ covalent radius = 99 pm.
Ionic radius: The effective radius of a cation or anion in a crystal lattice.
- Cations are smaller than their atoms (Na: 190 pm → Na⁺: 116 pm).
- Anions are larger than their atoms (F: 64 pm → F⁻: 136 pm).
Q3.10: How do atomic radius vary in a period and in a group? How do you explain the variation?
Ans: Within a group Atomic radius increases down the group.
Reason. This is due to continuous increases in the number of electronic shells or orbit numbers in the structure of atoms of the elements down a group.
Variation across period.
Atomic Radii. From left to right across a period atomic radii generally decreases due
to increase in effective nuclear charge from left to right across a period.
Q3.11: What do you understand by isoelectronic species? Name a species that will be isoelectronic with each of the following atoms or ions.
(i) F–
(ii) Ar
(iii) Mg2+
(iv) Rb+
Ans: Atoms and ions having the same number of electrons are called isoelectronic species.
(i) F– ion has 9 + 1 = 10 electrons. Thus, the species isoelectronic with it will also have 10 electrons. Some of its isoelectronic species are Na+ ion (11 – 1 = 10 electrons), Ne (10 electrons), O2– ion (8 + 2 = 10 electrons), and Al3+ ion (13 – 3 = 10 electrons).
(ii) Ar has 18 electrons. Thus, the species isoelectronic with it will also have 18 electrons. Some of its isoelectronic species are S2– ion (16+2 = 18 electrons), Cl– ion (17 + 1 = 18 electrons), K ion (19 – 1 = 18 electrons), and Ca2+ ion (20 – 2 = 18 electrons).
(iii) Mg2+ ion has 12 – 2 = 10 electrons. Thus, the species isoelectronic with it will also have 10 electrons. Some of its isoelectronic species are F– ion (9 +1 = 10 electrons), Ne (10 electrons), O2– ion (8 + 2 = 10 electrons), and Al3+ ion (13 – 3 = 10 electrons).
(iv) Rb+ ion has 37 – 1 = 36 electrons. Thus, the species isoelectronic with it will also have 36 electrons. Some of its isoelectronic species are Br– ion (35 + 1 = 36 electrons), Kr (36 electrons), and Sr2+ ion (38 – 2 = 36 electrons).
Q3.12: Consider the following species:
N3–, O2–, F–, Na+ , Mg2+ and Al3+
(a) What is common in them?
(b) Arrange them in the order of increasing ionic radii.
Ans: (a) Each of the given species (ions) has the same number of electrons (10 electrons). Hence, the given species are isoelectronic.
(b) The ionic radii of isoelectronic species increases with a decrease in the magnitudes of nuclear charge.
The arrangement of the given species in order of their increasing nuclear charge is as follows:
N3– < O2– < F– < Na+ < Mg2+ < Al3+
Nuclear charge = +7 + 8 +9 +11 +12 +13
Therefore, the arrangement of the given species in order of their increasing ionic radii is as follows:
Al3+ < Mg2+ < Na+ < F– < O2– < N3–
Q3.13: Explain why cations are smaller and anions larger in radii than their parent atoms?
Ans: A cation is smaller than the parent atom because it has fewer electrons while its nuclear charge remains the same. The size of anion will be larger than that of parent atom because the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge.
Q3.14: What is the significance of the terms - ‘isolated gaseous atom’ and ‘ground state’ while defining the ionization enthalpy and electron gain enthalpy?
Hint: Requirements for comparison purposes.
Ans: Ionization enthalpy is the energy required to remove an electron from an isolated gaseous atom in its ground state. Although the atoms are widely separated in the gaseous state, there are some amounts of attractive forces among the atoms. To determine the ionization enthalpy, it is impossible to isolate a single atom. But, the force of attraction can be further reduced by lowering the pressure. For this reason, the term ‘isolated gaseous atom’ is used in the definition of ionization enthalpy.
Ground state of an atom refers to the most stable state of an atom. If an isolated gaseous atom is in its ground state, then less amount energy would be required to remove an electron from it. Therefore, for comparison purposes, ionization enthalpy and electron gain enthalpy must be determined for an ‘isolated gaseous atom’ and its ‘ground state’.
Q3.15: Energy of an electron in the ground state of the hydrogen atom is –2.18 × 10–18 J. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol–1.
Ans: The ionisation enthalpy is for 1 mole atoms. Therefore, ground state energy of the , atoms may be expressed as E (ground state) = ( – 2.18 x 10-18 J) x(6.022 x 1023 mol-1)= -1.312 x 106 J mol-1
Ionisation enthalpy =E∞–E ground state
= 0-(-1.312 x 106mol-1)
= 1.312 x 106 J mol-1.
Q3.16: Among the second period elements the actual ionization enthalpies are in the order
Li < B < Be < C < O < N < F < Ne.
Explain why
(i) Be has higher ΔiH than B
(ii) O has lower ΔiH than N and F?
Ans: (i) In case of Be (1s2 2s2) the outermost electron is present in 2s-orbital while in B (1s2 2s2 2p1) it is present in 2p-orbital. Since 2s – electrons are more strongly attracted by the nucleus than 2p-electrons, therefore, lesser amount of energy is required to knock out a 2p-electron than a 2s – electron. Consequently, At of Be is higher than that ∆iH1 of B.
(ii) The electronic configuration of
N7 = 1s2 2s2 2px1 2py1 2pz1
O8 =1s2 2s2 2px1 2py1 2pz1
We can see that in case of nitrogen 2p-orbitals are exactly half filled. Therefore, it is difficult to remove an electron from N than from O. As a result ∆iH1 of N is higher than that of O.
NCERT Questions on Page No. 97
Q3.17: How would you explain the fact that the first ionization enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium?
Ans: The first ionization enthalpy of sodium is more than that of magnesium. This is primarily because of two reasons:
- The atomic size of sodium is greater than that of magnesium
- The effective nuclear charge of magnesium is higher than that of sodium
For these reasons, the energy required to remove an electron from magnesium is more than the energy required in sodium. Hence, the first ionization enthalpy of sodium is lower than that of magnesium.
However, the second ionization enthalpy of sodium is higher than that of magnesium. This is because after losing an electron, sodium attains the stable noble gas configuration. On the other hand, magnesium, after losing an electron still has one electron in the 3s-orbital. In order to attain the stable noble gas configuration, it still has to lose one more electron. Thus, the energy required to remove the second electron in case of sodium is much higher than that required in case of magnesium. Hence, the second ionization enthalpy of sodium is higher than that of magnesium.
Q3.18: What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
Ans: The factors responsible for the ionization enthalpy of the main group elements to decrease down a group are listed below:
(i) Increase in the atomic size of elements: As we move down a group, the number of shells increases. As a result, the atomic size also increases gradually on moving down a group. As the distance of the valence electrons from the nucleus increases, the electrons are not held very strongly. Thus, they can be removed easily. Hence, on moving down a group, ionization energy decreases.
(ii) Increase in the shielding effect: The number of inner shells of electrons increases on moving down a group. Therefore, the shielding of the valence electrons from the nucleus by the inner core electrons increases down a group. As a result, the valence electrons are not held very tightly by the nucleus. Hence, the energy required to remove a valence electron decreases down a group.
Q3.19: The first ionization enthalpy values (in kJmol–1) of group 13 elements are :
How would you explain this deviation from the general trend?
Ans: The decrease in ∆iH1 value from B to Al is due to the bigger size of Al.
In Ga there is 10 3d electrons which do not screen as is done by S and P electrons. Therefore, there is an unexpected increase in the magnitude of effective nuclear charge resulting in increased ∆iH1 values. The same is with into Tl. The later has fourteen ∆f electrons with very poor shielding effect. This also increases, the effective nuclear charge thus the value of ∆iH1 increases.
Q3.20: Which of the following pairs of elements would have a more negative electron gain enthalpy?
(i) O or F
(ii) F or Cl
Ans:
(i) O or F. Both O and F lie in 2nd period. As we move from O to F the atomic size decreases.
Due to smaller size of F nuclear charge increases.
Further, gain of one electron by
F —> F–
F~ ion has inert gas configuration, While the gain of one electron by
0->O–
gives CT ion which does not have stable inert gas configuration, consequently, the energy released is much higher in going from
F ->F–
than going from O —>O–
In other words electron gain enthalpy of F is much more negative than that of oxygen.
(ii) The negative electron gain enthalpy of Cl (∆ eg H = – 349 kj mol-1) is more than that of F (∆ eg H = – 328 kJ mol -1).
The reason for the deviation is due to the smaller size of F. Due to its small size, the electron repulsions in the relatively compact 2p-subshell are comparatively large and hence the attraction for incoming electron is less as in the case of Cl.
Q3.21: Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer.
Ans: For oxygen atom:
O (g) + e– —> O– (g) (∆ eg H = – 141 kJ mol -1)
O– (g) + e– —> O 2- (g) (∆ eg H = + 780 kJ mol -1)
The first electron gain enthalpy of oxygen is negative because energy is released when a gaseous atom accepts an electron to form monovalent anion. The second electron gain enthalpy is positive because energy is needed to overcome the force of repulsion between monovalent anion and second incoming electron.
Q3.22: What is the basic difference between the terms electron gain enthalpy and electronegativity?
Ans: Electron gain enthalpy refers to tendency of an isolated gaseous atom to accept an additional electron to form a negative ion. Whereas electronegativity refers to tendency of the atom of an element to attract shared pair of electrons towards it in a covalent bond.
Q3.23: How would you react to the statement that the electronegativity of N on Pauling scale is 3.0 in all the nitrogen compounds?
Ans: On Pauling scale, the electronegativity of nitrogen, (3.0) indicates that it is sufficiently electronegative. But it is not correct to say that the electronegativity of nitrogen in all the compounds is 3. It depends upon its state of hybridisation in a particular compound, greater the percentage of s-character, more will be the electronegativity of the element. Thus, the electronegativity of nitrogen increases in moving from SP3 hybridised orbitals to SP hybridised orbitals i.e., as SP3 < SP2 < SP.
Q3.24: Describe the theory associated with the radius of an atom as it
(a) gains an electron
(b) loses an electron
Ans:
- Gain of an electron leads to the formation of an anion. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased repulsion among electrons and decrease in effective nuclear charge.
This the ionic radius of fluoride ion (F–) is 136 pm whereas atomic radius of Fluorine (F) is only 64 pm. - Loss of an electron from an atom results in the formation of a cation. A cation is smaller than its parent atom because it has fomer electrons while its nuclear charge remains the same. For example, The atomic radius of sodium (Na) is 186 pm and atomic radius of sodium ion (Na+) = 95 pm.
Q3.25: Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different? Justify your answer.
Ans: The ionization enthalpy of an atom depends on the number of electrons and protons (nuclear charge) of that atom. Now, the isotopes of an element have the same number of protons and electrons. Therefore, the first ionization enthalpy for two isotopes of the same element should be the same.
Q3.26: What are the major differences between metals and non-metals?
Ans: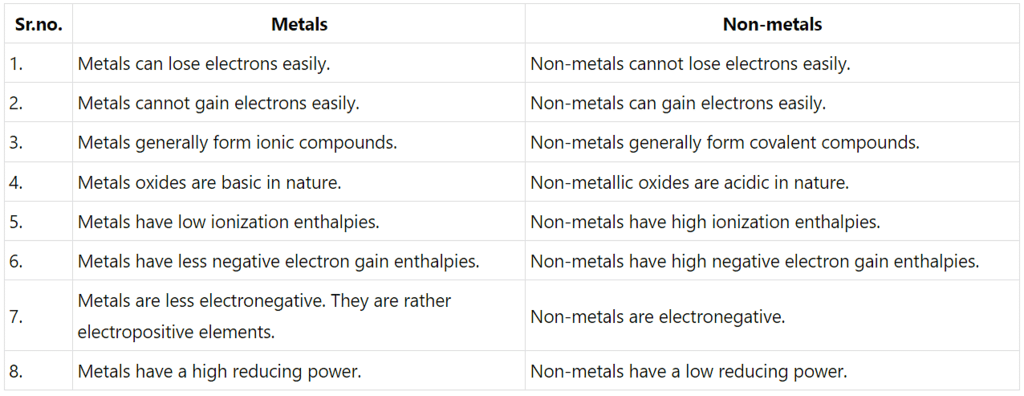
Q3.27: Use the periodic table to answer the following questions.
(a) Identify an element with five electrons in the outer subshell.
(b) Identify an element that would tend to lose two electrons.
(c) Identify an element that would tend to gain two electrons.
(d) Identify the group having metal, non-metal, liquid as well as gas at the room temperature.
Ans: ((a) Element belonging to nitrogen family (group 15) e.g., nitrogen.
(b) Element belonging to alkaline earth family (group 2) e.g., magnesium.
(c) Element belonging to oxygen family (group 16) e.g., oxygen.
Q3.28: The increasing order of reactivity among group 1 elements is Li < Na < K < Rb <Cs whereas that among group 17 elements is F > CI > Br > I. Explain.
Ans: The elements present in group 1 have only 1 valence electron, which they tend to lose. Group 17 elements, on the other hand, need only one electron to attain the noble gas configuration. On moving down group 1, the ionization enthalpies decrease. This means that the energy required to lose the valence electron decreases. Thus, reactivity increases on moving down a group. Thus, the increasing order of reactivity among group 1 elements is as follows:
Li < Na < K < Rb < Cs
In group 17, as we move down the group from Cl to I, the electron gain enthalpy becomes less negative i.e., its tendency to gain electrons decreases down group 17. Thus, reactivity decreases down a group. The electron gain enthalpy of F is less negative than Cl. Still, it is the most reactive halogen. This is because of its low bond dissociation energy. Thus, the decreasing order of reactivity among group 17 elements is as follows:
F > Cl > Br > I
Q3.29: Write the general outer electronic configuration of s-, p-, d- and f- block elements.
Ans: Element General outer electronic configuration
s–block : ns1–2, where n = 2 – 7
p–block: ns2np1–6, where n = 2 – 6
d–block: (n–1) d1–10 ns0–2, where n = 4 – 7
f–block: (n–2)f1–14(n–1)d0–10ns2, where n = 6 – 7
Q3.30: Assign the position of the element having outer electronic configuration
(i) ns2 np4 for n = 3
(ii) (n - 1)d2 ns2 for n = 4, and
(iii) (n - 2) f7 (n - 1)d1 ns2 for n = 6, in the periodic table.
Ans: (i) n = 3
Thus element belong to 3rd period, p-block element.
Since the valence shell contains = 6 electrons, group No = 10 + 6 = 16 configuration =1s2 2s2 2p6 3s2 3p4 element name is sulphur.
(ii) n = 4
Means element belongs to 4th period belongs to group 4 as in the valence shell (2 + 2) = 4 electrons.
Electronic configuration.=1s2 2s2 2p6 3s2 3p6 3d2 4s2, and the element name is Titanium (Ti).
(iii) n = 6
” Means the element belongs to 6th period. Last electron goes to the f-orbital, element is from f-block.
group = 3
The element is gadolinium (z = 64)
Complete electronic configuration =[Xe] 4 f7 5d1 6s2.
NCERT Questions on Page No. 98
Q3.31: The first (ΔiH1) and the second (ΔiH) ionization enthalpies (in kJ mol–1) and the (ΔegH) electron gain enthalpy (in kJ mol–1) of a few elements are given below: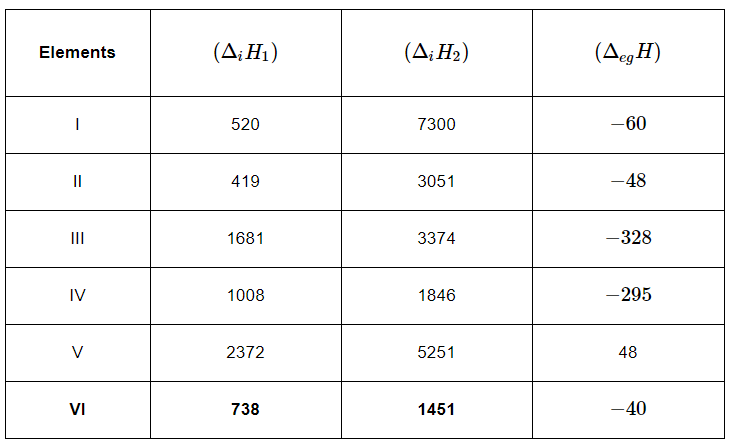
Which of the above elements is likely to be:
(a) the least reactive element.
(b) the most reactive metal.
(c) the most reactive non-metal.
(d) the least reactive non-metal.
(e) the metal which can form a stable binary halide of the formula MX2, (X=halogen).
(f) the metal which can form a predominantly stable covalent halide of the formula MX (X=halogen)?
Ans: (a) Element V is likely to be the least reactive element. This is because it has the highest first ionization enthalpy (ΔiH1) and a positive electron gain enthalpy (ΔegH).
(b) Element II is likely to be the most reactive metal as it has the lowest first ionization enthalpy (ΔiH1) and a low negative electron gain enthalpy (ΔegH).
(c) Element III is likely to be the most reactive non–metal as it has a high first ionization enthalpy (ΔiH1) and the highest negative electron gain enthalpy (ΔegH).
(d) Element V is likely to be the least reactive non–metal since it has a very high first ionization enthalpy (Δi H2) and a positive electron gain enthalpy (Δeg H).
(e) Element VI has a low negative electron gain enthalpy (Δeg H). Thus, it is a metal. Further, it has the lowest second ionization enthalpy (Δi H2). Hence, it can form a stable binary halide of the formula MX2 (X=halogen).
(f) Element I has low first ionization energy and high second ionization energy. Therefore, it can form a predominantly stable covalent halide of the formula MX (X=halogen).
Q3.32: Predict the formula of the stable binary compounds that would be formed by the combination of the following pairs of elements.
(a) Lithium and oxygen
(b) Magnesium and nitrogen
(c) Aluminium and iodine
(d) Silicon and oxygen
(e) Phosphorus and fluorine
(f) Element 71 and fluorine
Ans:- (a) LiO2
(b) Mg3N2
(c) AlI3
(d) SiO2
(e) PF3 or PF5
(f) The element with the atomic number 71 is Lutetium (Lu). It has valency 3. Hence, the formula of the compound is LuF3.
Q3.33: In the modern periodic table, the period indicates the value of:
(a) Atomic number
(b) Atomic mass
(c) Principal quantum number
(d) Azimuthal quantum number.
Ans: In the modern periodic table, each period begins with the filling of a new shell. Therefore, the period indicates the value of principal quantum number. Thus, option (c) is correct.
Q3.34: Which of the following statements related to the modern periodic table is incorrect?
(a) The p-block has 6 columns, because a maximum of 6 electrons can occupy all the orbitals in a p-shell.
(b) The d-block has 8 columns, because a maximum of 8 electrons can occupy all the orbitals in a d-subshell.
(c) Each block contains a number of columns equal to the number of electrons that can occupy that subshell.
(d) The block indicates value of azimuthal quantum number (l ) for the last subshell that received electrons in building up the electronic configuration.
Ans: The d-block has 10 columns because a maximum of 10 electrons can occupy all the orbitals in a d subshell. Statement (b) is incorrect.
NCERT Questions on Page No. 99
Q3.35: Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z)
(c) Nuclear mass
(d) Number of core electrons.
Ans: (c) Nuclear mass does not affect the valence electrons
Q3.36: The size of isoelectronic species — F–, Ne and Na+ is affected by
(a) Nuclear charge (Z )
(b) Valence principal quantum number (n)
(c) Electron-electron interaction in the outer orbitals
(d) None of the factors because their size is the same.
Ans: The size of an isoelectronic species increases with a decrease in the nuclear charge (Z). For example, the order of the increasing nuclear charge of F–, Ne, and Na is as follows:
F– < Ne < Na+
Z = 9 10 11
Therefore, the order of the increasing size of F–, Ne and Na is as follows:
Na+ < Ne < F–
Q3.37: Which one of the following statements is incorrect in relation to ionization enthalpy?
(a) Ionization enthalpy increases for each successive electron.
(b) The greatest increase in ionization enthalpy is experienced on removal of electron from core noble gas configuration.
(c) End of valence electrons is marked by a big jump in ionization enthalpy.
(d) Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value.
Ans: Electrons in orbitals bearing a lower n value are more attracted to the nucleus than electrons in orbitals bearing a higher n value. Hence, the removal of electrons from orbitals bearing a higher n value is easier than the removal of electrons from orbitals having a lower n value.
Q3.38: Considering the elements B, Al, Mg, and K, the correct order of their metallic character is:
(a) B > Al > Mg > K
(b) Al > Mg > B > K
(c) Mg > Al > K > B
(d) K > Mg > Al > B
Ans: The metallic character of elements decreases from left to right across a period. Thus, the metallic character of Mg is more than that of Al.
The metallic character of elements increases down a group. Thus, the metallic character of Al is more than that of B.
Considering the above statements, we get K > Mg.
Hence, the correct order of metallic character is K > Mg > Al > B, i.e., option (d) is correct.
Q3.39: Considering the elements B, C, N, F, and Si, the correct order of their non-metallic character is:
(a) B > C > Si > N > F
(b) Si > C > B > N > F
(c) F > N > C > B > Si
(d) F > N > C > Si > B
Ans: The non-metallic character of elements increases from left to right across a period. Thus, the decreasing order of non-metallic character is F > N > C > B.
Again, the non-metallic character of elements decreases down a group. Thus, the decreasing order of non-metallic characters of C and Si are C > Si. However, Si is less non-metallic than B i.e., B > Si.
Hence, the correct order of their non-metallic characters is F > N > C > B > Si, i.e., option (c) is correct.
Q3.40: Considering the elements F, Cl, O and N, the correct order of their chemical reactivity in terms of oxidizing property is:
(a) F > Cl > O > N
(b) F > O > Cl > N
(c) Cl > F > O > N
(d) O > F > N > Cl
Ans: The oxidizing character of elements increases from left to right across a period. Thus, we get the decreasing order of oxidizing property as F > O > N.
Again, the oxidizing character of elements decreases down a group. Thus, we get F > Cl. However, the oxidizing character of O is more than that of Cl i.e., O > Cl.
Hence, the correct order of chemical reactivity of F, Cl, O, and N in terms of their oxidizing property is F > O > Cl > N, i.e., option (b) is correct.
|
119 videos|338 docs|74 tests
|
FAQs on NCERT Solutions Class 11 Chemistry Chapter 3 - Classification of Elements & Periodicity in Properties
| 1. What is the significance of the periodic table in the classification of elements? |  |
| 2. How does the periodicity of properties affect the reactivity of elements? |  |
| 3. What are the main groups of elements in the periodic table, and how are they classified? |  |
| 4. What trends can be observed in atomic size across periods and groups? |  |
| 5. How do the concepts of electronegativity and ionization energy relate to the periodic table? |  |





















