Class 8 Exam > Class 8 Notes > Science Curiosity Class 8 - New NCERT > NCERT Based Activity: Nature of Matter: Elements, Compounds, and Mixtures
NCERT Based Activity: Nature of Matter: Elements, Compounds, and Mixtures | Science Curiosity Class 8 - New NCERT PDF Download
Activity 8.1: Let us experiment
- Take a glass tumbler and fill it half with water.
- Add a small amount of calcium oxide (quick lime) slowly to it.
- What do you observe?
- Stir continuously to make a solution of calcium hydroxide. This solution is called lime water.
- Filter it and observe its colour.
- Leave this colourless solution in a petri dish for a few hours (Fig. 8.5a).
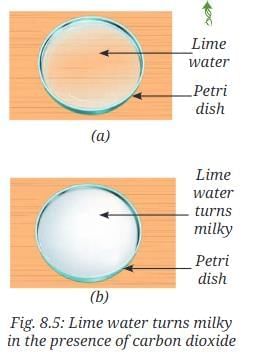
- Keep stirring the solution at regular intervals.
- What do you observe (Fig. 8.5b)?
- Does it turn milky?
- Can you explain why the solution has turned milky?
Answer:
- Calcium oxide reacts vigorously with water, releasing heat and forming calcium hydroxide.
- The filtered lime water is initially colourless. After a few hours, it turns milky (Fig. 8.5b).
- This occurs because carbon dioxide in the air reacts with calcium hydroxide to form insoluble calcium carbonate and water, indicating the presence of carbon dioxide in the air.
Activity 8.2: Let us explore
- Take a black sheet of paper. Ensure that it is free from any visible dust particles.
- Place the black sheet of paper undisturbed near an open window (Fig. 8.6a), or in the garden, for a few hours.
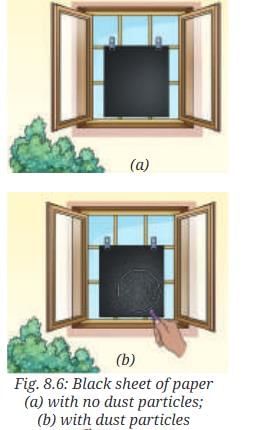
- What do you observe?
Answer:
- Tiny particles settle on the surface of the black sheet of paper.
- Using a magnifying glass (Fig. 8.6b), these can be identified as dust particles suspended in the air, which are considered pollutants and not an integral part of the air.
Activity 8.3: Let us experiment (Demonstration activity)
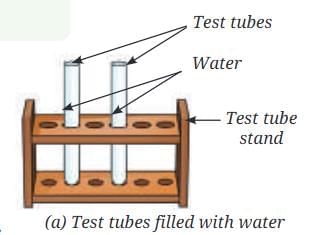
- Collect two small test tubes, a beaker or a glass tumbler, and a 9 V battery.
- Fill 2/3rd of the beaker with water and add a few drops of dilute sulfuric acid to it.
- Fill both the small test tubes completely with water taken from the beaker (Fig. 8.8a).
- Place a 9 V battery inside the beaker (Fig. 8.8b).
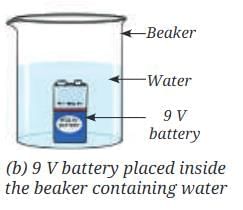
- Without spilling the water, carefully place the water-filled test tubes on each of the terminals of the battery (Fig. 8.8c).
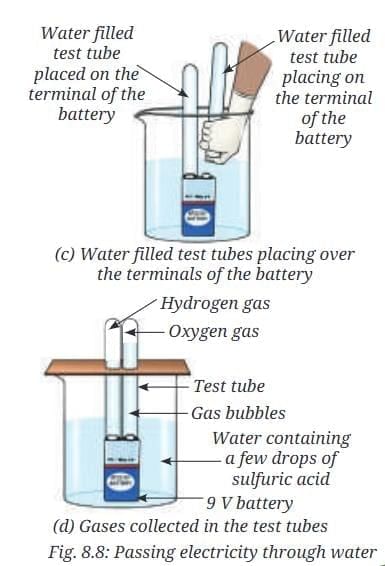
- Wait for a few minutes.
- Do you observe the formation of any gas bubbles at both the terminals inside the test tubes?
- Let it continue for 10–15 minutes.
- Observe the volume of gas collected in each test tube (Fig. 8.8d).
- Is the volume of the gas collected the same in both the test tubes?
- Remove these test tubes one-by-one carefully.
- Test these gases one-by-one by bringing a burning candle close to the mouth of the test tubes.
- What happens in each case?
- Which gas is present in each test tube?
- Can these collected gases be water vapour?
Answer:
- Gas bubbles form at both terminals inside the test tubes.
- After 10–15 minutes, the volume of gas collected differs, with one test tube containing more gas than the other.
- Testing with a burning candle produces a pop sound in one test tube (indicating hydrogen gas) and a brighter flame in the other (indicating oxygen gas).
- The volumes are not the same, typically in a 2:1 ratio (hydrogen:oxygen).
- These gases are not water vapour, as they do not condense back into water but are elements formed by the breakdown of water into hydrogen and oxygen.
Activity 8.4: Let us experiment
- Put a teaspoon of sugar in a boiling tube.
- Heat it gently as shown in Fig. 8.12a.
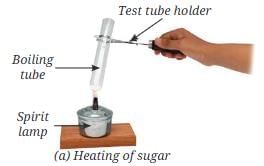
- What do you observe?
Answer:
- As sugar is heated, it turns brown (Fig. 8.12b), then chars and turns blackish (Fig. 8.12c).
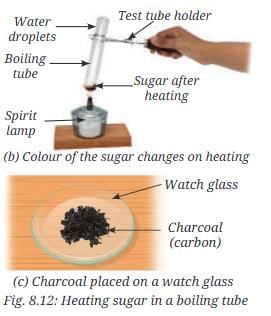
- Small droplets of water form inside the boiling tube near the open end, indicating that sugar decomposes into carbon (charcoal) and water (which contains hydrogen and oxygen).
- This shows sugar is a compound, not an element.
Activity 8.5: Let us experiment (Demonstration activity)
- Take 5.6 grams of iron filings (Fig. 8.13a) and 3.2 grams of sulfur powder (Fig. 8.13b) on a watch glass. Observe them carefully.

- Mix them thoroughly in a watch glass. Label this mixture as Sample A (Fig. 8.14).
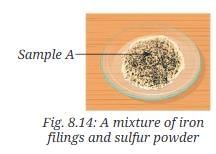
- Observe it carefully.
- Is this a uniform or a non-uniform mixture? Can you still observe both iron and sulfur as separate substances?
- Take half of Sample A in a china dish and gently heat it (Fig. 8.15a) with continuous stirring until a black mass is formed.
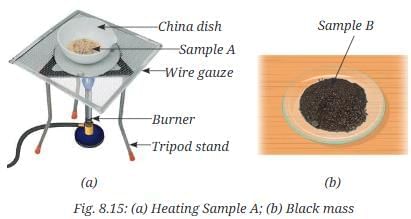
- Let the content of the china dish cool.
- Place this black mass in a mortar and grind it with the help of a pestle.
- Now, put it on another watch glass and label it as Sample B (Fig. 8.15b).
- Now, you have two samples—Sample A and Sample B (Fig. 8.16a and 8.16b). Compare both the Samples A and B step by step and record your observations in Table 8.2.

- Step 1—Appearance: Compare the appearance of Sample A and Sample B like colour and texture.
- Step 2—Magnet test: Take a magnet and move it over the Samples A (Fig. 8.17a) and B (Fig. 8.17b), one by one.
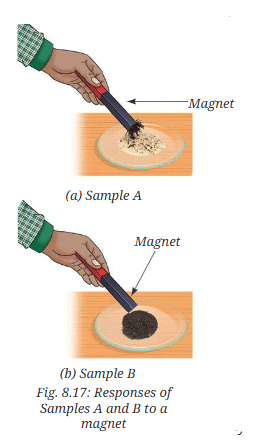
- What do you observe?
- Step 3—Gas test: Take a small amount of Sample A in a test tube and add a few drops of dilute hydrochloric acid (Fig. 8.18a).
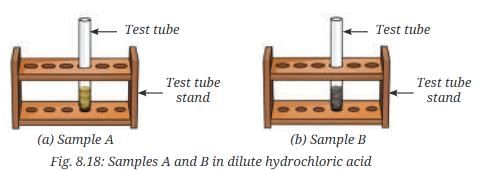
- What do you observe?
- Gently smell the evolved gas by wafting it towards your nose (Fig. 8.19).

- Test the evolved gas by bringing a burning splinter or a lighted candle near the mouth of the test tube (Fig. 8.20a).
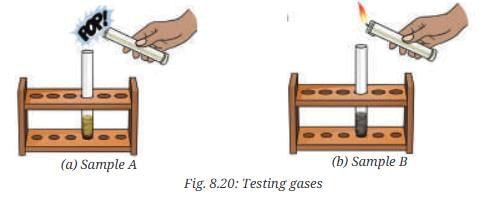
- What do you observe?
- Repeat the above steps with Sample B as well (Fig. 8.18b and 8.20b).
Answer:
- Sample A is a non-uniform mixture where iron and sulfur particles are visible separately. On heating, Sample A forms a black mass (Sample B), which is uniform in color and texture.
- The magnet attracts iron filings in Sample A but not in Sample B. In the gas test, Sample A produces hydrogen gas (colorless, no smell, pops with a flame) due to iron reacting with hydrochloric acid, while sulfur remains unreacted.
- Sample B produces hydrogen sulfide gas (colorless, rotten egg-like odor) when reacted with hydrochloric acid, indicating a compound (iron sulfide) with different properties from the original mixture.
The document NCERT Based Activity: Nature of Matter: Elements, Compounds, and Mixtures | Science Curiosity Class 8 - New NCERT is a part of the Class 8 Course Science Curiosity Class 8 - New NCERT.
All you need of Class 8 at this link: Class 8
|
59 videos|236 docs|13 tests
|
FAQs on NCERT Based Activity: Nature of Matter: Elements, Compounds, and Mixtures - Science Curiosity Class 8 - New NCERT
| 1. What are elements, compounds, and mixtures? |  |
Ans.Elements are pure substances that consist of only one type of atom, such as hydrogen (H) or oxygen (O). Compounds are substances formed when two or more elements chemically combine in fixed ratios, like water (H₂O) or carbon dioxide (CO₂). Mixtures are combinations of two or more substances that retain their individual properties, such as air or salad.
| 2. How can we differentiate between a compound and a mixture? |  |
Ans.The main difference between a compound and a mixture is that compounds are formed through chemical reactions and have a fixed composition, whereas mixtures can be separated by physical methods and do not have a fixed composition. For example, saltwater is a mixture of salt and water that can be separated by evaporation, while sodium chloride (table salt) is a compound that cannot be separated by simple physical means.
| 3. What are some common methods to separate mixtures? |  |
Ans.Common methods to separate mixtures include filtration, which separates solids from liquids; distillation, which separates liquids based on differences in boiling points; and chromatography, which separates substances based on their movement through a medium. Each method utilizes the physical properties of the components in the mixture.
| 4. Why is understanding the nature of matter important? |  |
Ans.Understanding the nature of matter is crucial because it forms the basis of chemistry and helps us comprehend the material world. It allows us to categorize substances, understand their properties and reactions, and apply this knowledge in various fields such as medicine, engineering, and environmental science.
| 5. What experiments can illustrate the concepts of elements, compounds, and mixtures? |  |
Ans.Experiments such as creating a simple solution (like saltwater) to demonstrate a mixture, reacting vinegar with baking soda to form a compound (carbon dioxide gas), or separating a mixture of sand and salt using water and filtration can effectively illustrate these concepts. These hands-on activities help reinforce theoretical knowledge through practical application.
Related Searches





















