Unit Test (Solutions): The Amazing World of Solutes, Solvents, and Solutions | Science Class 8 PDF Download
Time: 1 hour
M.M. 30
Attempt all questions.
- Question numbers 1 to 5 carry 1 mark each.
- Question numbers 6 to 8 carry 2 marks each.
- Question numbers 9 to 11 carry 3 marks each.
- Question numbers 12 & 13 carry 5 marks each.
- 1-mark questions include MCQs.
Q1: A solution is defined as (1 Mark)
(i) any mixture of two solids
(ii) a uniform mixture in which components are evenly distributed
(iii) a non-uniform mixture where components settle
(iv) only liquids mixed with liquids
Ans: (ii)
A solution is a uniform (homogeneous) mixture; for example, salt or sugar dissolved in water gives the same taste in every sip due to even distribution of particles.
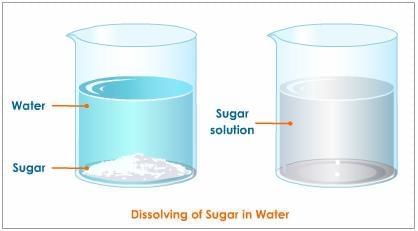
Q2: In the solution of salt in water, salt is the ______ and water is the ______. (1 Mark)
(i) solvent, solute
(ii) solute, solvent
(iii) suspension, colloid
(iv) liquid, solid
Ans: (ii)
The component present in smaller amount that dissolves is the solute (salt); the component present in larger amount that dissolves the solute is the solvent (water).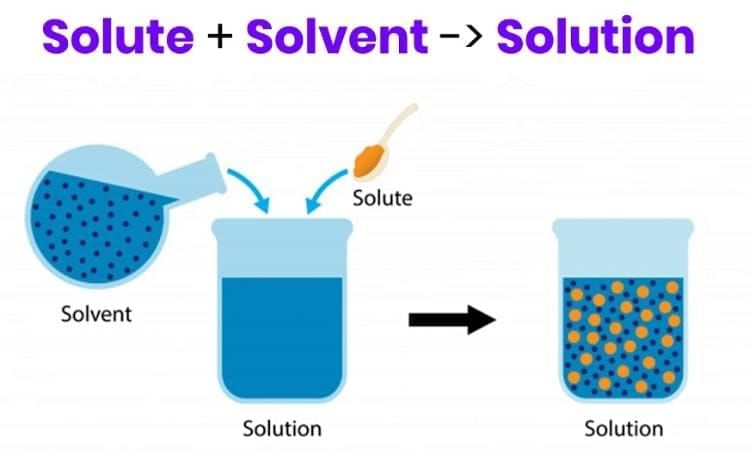
Q3: When no more solute can dissolve in a solution at a given temperature, the solution is (1 Mark)
(i) dilute
(ii) concentrated
(iii) saturated
(iv) supersaturated at all temperatures
Ans: (iii)
A saturated solution contains the maximum amount of solute dissolved at that temperature; any extra solute remains undissolved.
Q4: Generally, the solubility of most solids in liquids ______ with increase in temperature, while the solubility of gases in liquids ______. (1 Mark)
(i) decreases, increases
(ii) increases, decreases
(iii) unchanged, unchanged
(iv) decreases, decreases
Ans: (ii)
For most solids (e.g., baking soda), solubility increases with temperature; gases (e.g., oxygen) dissolve less in warmer water and more in colder water.
Q5: Density is defined as (1 Mark)
(i) mass × volume
(ii) volume/mass
(iii) mass/volume
(iv) weight/area
Ans: (iii)
Density = mass divided by volume; units commonly used in this chapter are g/cm3 or g/mL for solids and liquids.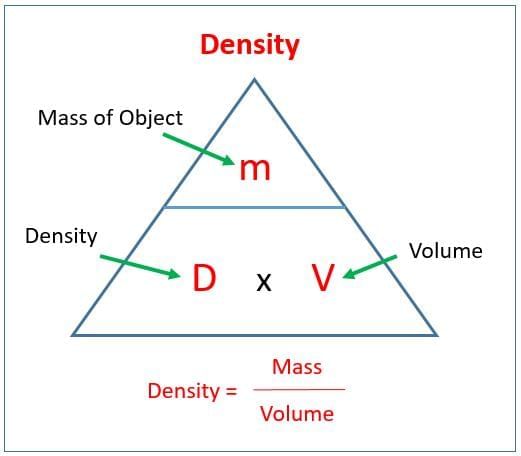
Q6: State the difference between a saturated and an unsaturated solution with one example from class activities. (2 Marks)
Ans: A saturated solution has dissolved the maximum solute possible at a given temperature (e.g., salt in water with undissolved crystals at the bottom). An unsaturated solution can still dissolve more solute at that temperature (e.g., first few spoonfuls of salt in water dissolve completely).
Q7: Give one reason why air is considered a solution. Identify the solvent in air. (2 Marks)
Ans: Air is a uniform mixture of gases (homogeneous), hence a gaseous solution. Nitrogen, being present in the largest amount, acts as the solvent; oxygen, argon, CO2, etc., act as solutes.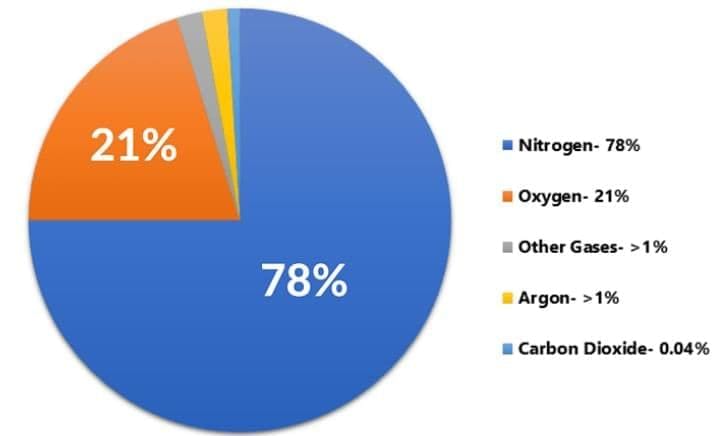
Q8: Explain why oxygen dissolves more in cold water than in hot water. How is this important for aquatic life? (2 Marks)
Ans: Gas solubility generally decreases with increasing temperature; cold water holds more dissolved oxygen than warm water. Aquatic organisms rely on this dissolved oxygen—colder waters support more oxygen availability for fish and plants.
Q9: In an experiment, baking soda is added to water at 20 °C until some remains undissolved. On heating to 50 °C, the undissolved part disappears. Explain what this shows about solubility and the status of the solution at the higher temperature. (3 Marks)
Ans: This shows that solubility of the solid increases with temperature. The solution that was saturated at 20 °C becomes unsaturated at 50 °C, allowing more solute to dissolve until a new saturation level is reached at the higher temperature.
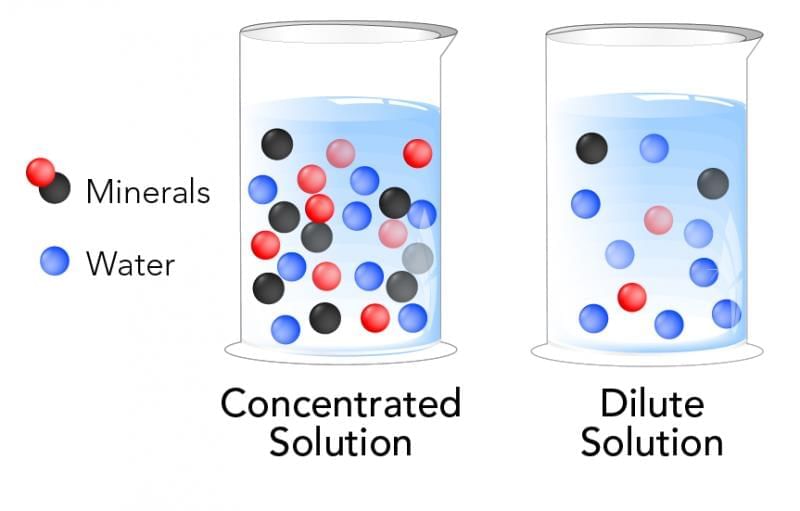
Q10: Define concentration in simple terms. Which is more concentrated and why: (a) 2 spoons of salt in 100 mL water or (b) 4 spoons of salt in 50 mL water? (3 Marks)
Ans: Concentration describes how much solute is present in a given amount of solution/solvent. Comparing (a) and (b), the same “spoon” implies same solute amount per spoon; (b) has double solute in half the volume, so (b) is much more concentrated.
Q11: A student mixes sawdust in water and notices it floats, while sand sinks. Use density ideas to explain both observations. (3 Marks)
Ans: Floating or sinking depends partly on relative density to water. Sawdust is less dense than water, so it floats. Sand is denser than water, so it sinks. Shape and trapped air can also influence floating, but intrinsic density provides the primary explanation here.
Q12: Measurement and density applications. (5 Marks)
(a) Describe how to measure 50 mL of water accurately in a measuring cylinder (mention meniscus and eye position).
(b) A stone has mass 16.400 g. Using water displacement, its volume increases from 50 mL to 55 mL after immersion. Calculate its density.
(c) Predict qualitatively how heating a liquid generally affects its density and why.
Ans:
(a) Place the measuring cylinder on a flat surface; pour water near the 50 mL mark; read at eye level with the bottom of the meniscus aligned to the 50 mL line (for colourless liquids). Avoid parallax error.
(b) Displaced volume = 55 mL − 50 mL = 5 mL = 5 cm3. Density = mass/volume = 16.400 g / 5 cm3 = 3.28 g/cm3.
(c) On heating, particles move further apart; volume increases while mass is constant, so density (mass/volume) generally decreases. Hence warm liquids are typically less dense than cold ones.
Q13: (a) Give one everyday example showing that solutions can be formed from gases too; identify solute and solvent.
(b) An unpeeled orange floats while a peeled one sinks. Explain.
(c) A 2 L bottle currently holds 500 mL of water. How much more water can it hold? If filled completely, what mass of water would it contain approximately at room temperature? (5 Marks)
Ans:
(a) Air is a gaseous solution: nitrogen is the solvent (largest proportion); oxygen, argon, carbon dioxide, etc., are solutes distributed uniformly.
(b) The unpeeled orange has a porous peel trapping air, lowering the average density below that of water, so it floats. Removing the peel removes the trapped air and increases average density, so the peeled orange sinks.
(c) Remaining capacity = 2000 mL − 500 mL = 1500 mL = 1.5 L. At room temperature, 1 mL water ≈ 1 g, so a full 2 L (2000 mL) bottle contains ≈ 2000 g = 2.0 kg of water.
|
136 videos|530 docs|57 tests
|
FAQs on Unit Test (Solutions): The Amazing World of Solutes, Solvents, and Solutions - Science Class 8
| 1. What is the difference between a solute and a solvent in a solution? |  |
| 2. How do temperature and pressure affect the solubility of substances? |  |
| 3. What are some common examples of solutions in everyday life? |  |
| 4. What is saturation in the context of solutions? |  |
| 5. How can we separate solutes from solvents in a solution? |  |















