Unit Test Solutions: Nature of Matter: Elements, Compounds, and Mixtures | Science Curiosity Class 8 - New NCERT PDF Download
Time: 1 hour
M.M. 30
Attempt all questions.
- Question numbers 1 to 5 carry 1 mark each.
- Question numbers 6 to 8 carry 2 marks each.
- Question numbers 9 to 11 carry 3 marks each.
- Question numbers 12 & 13 carry 5 marks each.
- 1-mark questions include MCQs.
Q1: Air is best described as (1 Mark)
(i) an element
(ii) a compound
(iii) a uniform (homogeneous) mixture
(iv) a non-uniform (heterogeneous) mixture
Ans: (iii)
Air is a uniform mixture of mainly nitrogen, oxygen, argon, carbon dioxide, and water vapour; components are mixed evenly and retain their identities.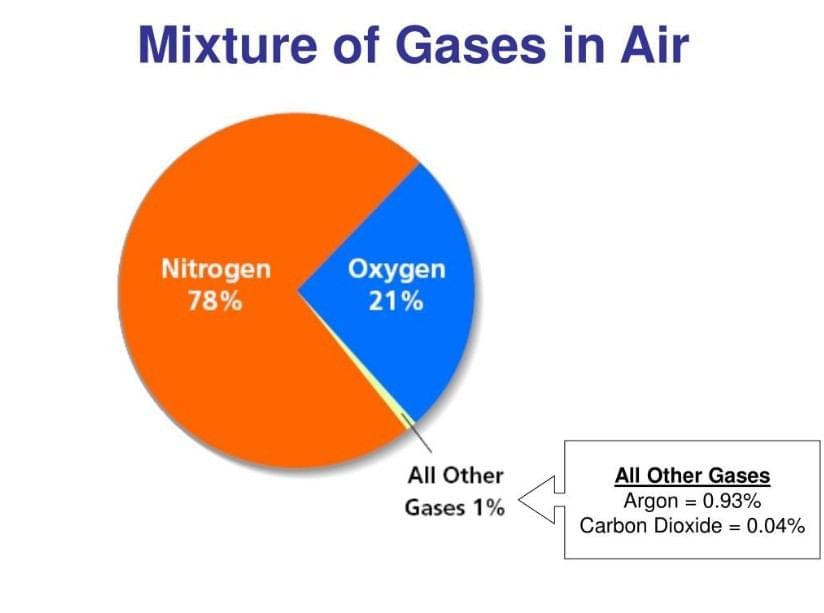
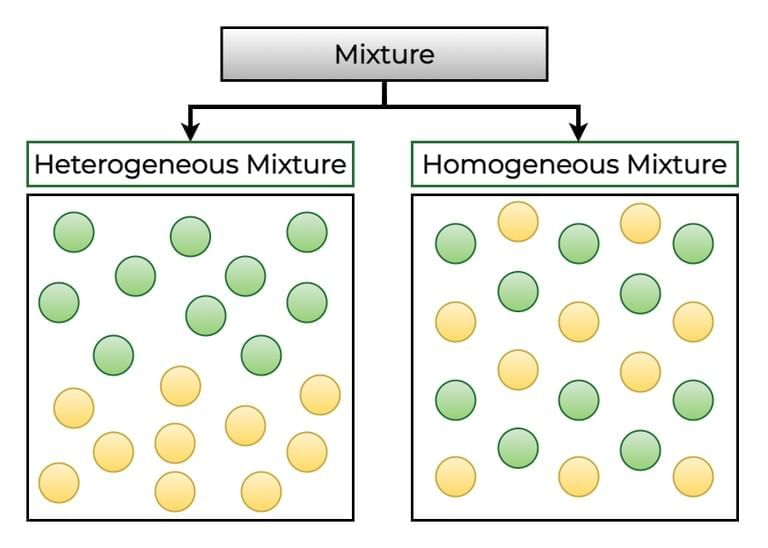
Q2: A mixture is formed when two or more substances are combined such that (1 Mark)
(i) they chemically react to form a new substance
(ii) each component retains its properties and can be separated by physical means
(iii) the components necessarily have fixed ratios
(iv) the components are always visible
Ans: (ii)
Mixture components do not chemically react and can be separated by suitable physical processes; their ratios may vary and visibility depends on type.
Q3: Which of the following is a pure substance in the scientific sense? (1 Mark)
(i) Seawater
(ii) Air
(iii) Oxygen gas
(iv) Milk
Ans: (iii)
Oxygen consists of identical particles (O2 molecules) and cannot be separated into simpler substances by physical methods; seawater, air, milk are mixtures.
Q4: When water is decomposed by passing electricity, the products are (1 Mark)
(i) hydrogen and oxygen
(ii) hydrogen only
(iii) oxygen only
(iv) hydrogen and carbon dioxide
Ans: (i)
Electrolysis of water yields hydrogen (pop sound test) and oxygen (supports brighter flame), revealing that water is a compound of H and O.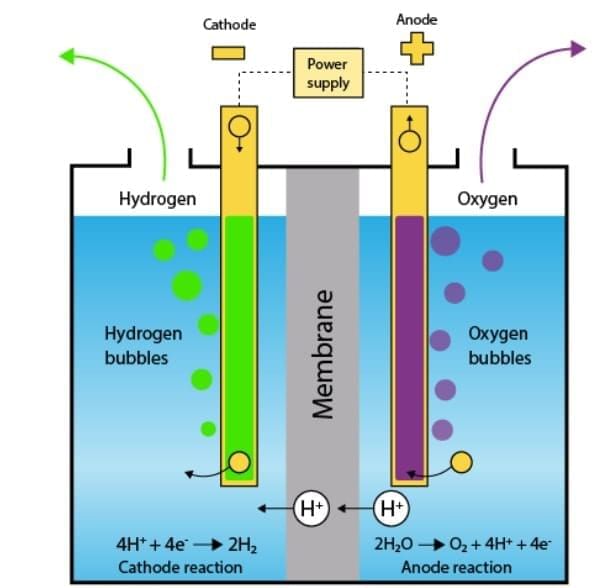 Electrolysis of Water
Electrolysis of Water
Q5: Which statement about compounds is correct? (1 Mark)
(i) Components are present in any proportion and retain their properties.
(ii) Components are present in fixed ratios and the compound has new properties.
(iii) Components can be separated by filtration and evaporation only.
(iv) Compounds are always gases.
Ans: (ii)
In compounds, elements combine chemically in fixed ratios producing substances with properties different from constituent elements; they require chemical means for separation.
Q6: Give one everyday example each of a uniform mixture and a non-uniform mixture; explain why. (2 Marks)
Ans: Uniform: Sugar in water—looks the same throughout; sugar particles are evenly distributed and not visible separately.
Non-uniform: Sprout salad—grains, onion, tomato are distinct and unevenly distributed, visible by eye.
Q7: “Pure” in everyday language vs “pure” in science—differentiate with one example. (2 Marks)
Ans: Everyday “pure” means unadulterated (e.g., “pure” milk without added water). Scientific “pure” means a single substance with identical particles (e.g., distilled water); milk is a mixture scientifically, not a pure substance.
Q8: Classify stainless steel and brass; justify your answer. (2 Marks)
Ans: Both are alloys—uniform mixtures of metals. Stainless steel: iron+chromium+nickel(+carbon) mixed uniformly; brass: copper+zinc. They are homogeneous mixtures, not compounds, since composition can vary and components retain metallic nature.
Q9: In the “lime water” activity, lime water turns milky when exposed to air. Explain the observation and write the word equation. (3 Marks)
Ans: Air contains carbon dioxide which reacts with calcium hydroxide solution (lime water) to form insoluble calcium carbonate causing milkiness and water.
Word equation: Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water.
Q10: Consider the mixture of iron filings and sulfur (Sample A) and the heated product (Sample B). Describe two tests that distinguish them and the inferences. (3 Marks)
Ans:
(i) Magnet test: Sample A is attracted (iron filings retain magnetism); Sample B is not attracted—indicates new substance formed where iron’s property is not retained.
(ii) Dilute HCl test: Sample A—iron reacts to release hydrogen (colourless, pop sound); sulfur remains unreacted. Sample B—iron sulfide reacts to produce hydrogen sulfide gas (rotten egg smell). The different gases indicate Sample B is a compound with new properties.
Q11: A student says, “Sugar is an element because it looks uniform.” Correct this misconception using evidence from heating sugar in a test tube. (3 Marks)
Ans: On heating sugar, it first browns then chars, leaving carbon (charcoal) and producing water droplets (from hydrogen and oxygen in sugar). This decomposition into simpler substances shows sugar is not an element but a compound of carbon, hydrogen, and oxygen.
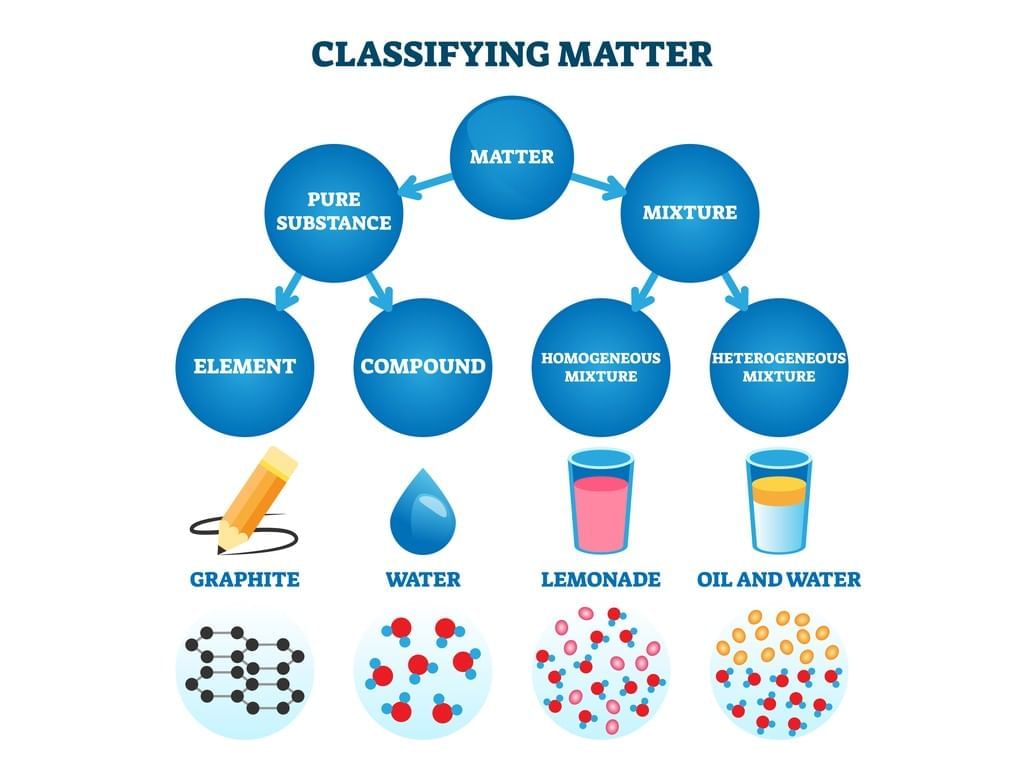
Q12: (a) Define element, compound, and mixture with one example each.
(b) Why can’t the elements of a compound be separated by physical methods, whereas mixture components can?
(c) For water, state the fixed ratio and contrast its properties with hydrogen and oxygen. (5 Marks)
Ans:
(a) Element: Simplest pure substance made of identical atoms; e.g., oxygen (O2).
Compound: Pure substance of two or more elements chemically combined in fixed ratio; e.g., water (H and O).
Mixture: Combination of substances retaining their properties; e.g., air (N2, O2, etc.).
(b) In compounds, elements are chemically bonded; separation requires chemical changes (e.g., electrolysis). Mixture components are not chemically combined and can be separated by physical methods (filtration, distillation, evaporation, magnet, etc.).
(c) Water has H:O atom ratio 2:1. Hydrogen is a flammable gas (burns with pop); oxygen supports combustion; water extinguishes fire and is liquid at room temperature—showing new properties distinct from its elements.
Q13: Extended reasoning and classification.
(a) Explain why air quality measurements report “particulate matter” although air is a uniform mixture.
(b) Classify each as element, compound, or mixture, with brief justification: oxygen, sodium chloride, seawater, iron sulfide, muddy water, glucose, brass, nitrogen, baking soda, sand.
(c) Using Below Figure: iron + dilute HCl → Gas A), identify Gas A and write the word equation. (5 Marks)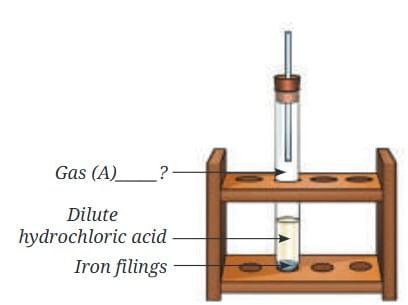
Ans:
(a) Air is a homogeneous mixture of gases, but can carry suspended solid/liquid pollutant particles (PM). These particulates are not part of the gaseous mixture’s composition and vary by place/time, so AQI reports them separately.
(b) Oxygen—element (single kind of particle O2);
Sodium chloride—compound (Na and Cl in fixed 1:1);
Seawater—mixture (water + dissolved salts, variable);
Iron sulfide—compound (new properties vs Fe/S, fixed ratio);
Muddy water—mixture (non-uniform, solids suspended);
Glucose—compound (C, H, O in fixed ratio);
Brass—mixture (Cu+Zn alloy, variable composition);
Nitrogen—element (N2);
Baking soda—compound (sodium hydrogen carbonate);
Sand—mixture in common use or mainly compound SiO2 at particle level; as a sample with impurities, treated as mixture.
(c) Gas A is hydrogen—observed by the ‘pop’ sound when tested with a flame.
Word equation: Iron + Dilute hydrochloric acid → Iron chloride + Hydrogen.
|
59 videos|236 docs|13 tests
|
FAQs on Unit Test Solutions: Nature of Matter: Elements, Compounds, and Mixtures - Science Curiosity Class 8 - New NCERT
| 1. What are the main differences between elements, compounds, and mixtures? |  |
| 2. How do elements and compounds differ in terms of their properties? |  |
| 3. Can mixtures be classified into different types? If so, what are they? |  |
| 4. What role does the Law of Conservation of Mass play in chemical reactions involving compounds? |  |
| 5. How can one separate the components of a mixture? |  |
















