NCERT Textbook Solutions: Nature of Matter: Elements, Compounds, and Mixtures | Science Class 8 PDF Download
Probe and Ponder

1. Which of the entities in the picture above consist of matter, and which of them do not?
Ans:
- Entities consisting of matter include physical objects like staircases, air, water, food, clothes, shoes, books, trees, balls, and sticks, as these have mass and occupy space.
- They are made of tiny particles. Entities that do not consist of matter include light, heat, electricity, thoughts, and emotions, as they lack mass and do not occupy space.
2. How can elements be combined to form a compound?
Ans:
- Elements combine chemically in fixed ratios to form compounds.
- For example, hydrogen and oxygen combine in a 2:1 ratio to form water, where the atoms bond tightly, creating a new substance with properties different from the original elements.
- This requires a chemical reaction, not just physical mixing.
3. How could the discovery of a compound that absorbs carbon dioxide from the air contribute to solving environmental challenges?
Ans:
- Such a compound could reduce atmospheric carbon dioxide levels, mitigating global warming and climate change.
- For instance, it might be used in technologies to capture emissions from industries or vehicles, similar to how calcium hydroxide reacts with carbon dioxide to form calcium carbonate.
- This could help address air pollution and support environmental cleanup efforts.
4. Share your questions
Ans: Based on the chapter, potential questions could include:
- What happens when elements like iron and sulfur are heated together?
- Why does water extinguish fire while its components (hydrogen and oxygen) support combustion?
- How do alloys like stainless steel improve everyday materials?
Keep the Curiosity Alive
Q1. Consider the following reaction where two substances, A and B, combine to form a product C: A + B → C. Assume that A and B cannot be broken down into simpler substances by chemical reactions. Based on this information, which of the following statements is correct?
(i) A, B, and C are all compounds and only C has a fixed composition.
(ii) C is a compound, and A and B have a fixed composition.
(iii) A and B are compounds, and C has a fixed composition.
(iv) A and B are elements, C is a compound, and has a fixed composition.
Ans: (iv) A and B are elements, C is a compound, and has a fixed composition.
This is because elements are simplest substances that cannot be broken down further, and they combine chemically in fixed ratios to form compounds with new properties.
Q2. Assertion: Air is a mixture.
Reason: A mixture is formed when two or more substances are mixed, without undergoing any chemical change.
(i) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
(ii) Both Assertion and Reason are true, but Reason is not the correct explanation for Assertion.
(iii) Assertion is true, but Reason is false.
(iv) Assertion is false, but Reason is true
Ans: (i) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
Air consists of gases like nitrogen, oxygen, argon, carbon dioxide, and water vapor mixed without chemical reaction, retaining their individual properties.
Q3. Water, a compound, has different properties compared to those of the elements oxygen and hydrogen from which it is formed. Justify this statement.
Ans:
- Water is formed by hydrogen and oxygen combining in a 2:1 ratio through a chemical reaction.
- Hydrogen is a flammable gas, oxygen supports combustion, but water is a liquid that extinguishes fire.
- This shows compounds have properties distinct from their constituent elements, where water decomposes into hydrogen and oxygen via electrolysis.
Q4. In which of the following cases are all the examples correctly matched? Give reasons in support of your answers.
(i) Elements — water, nitrogen, iron, air.
(ii) Uniform mixtures— minerals, seawater, bronze, air.
(iii) Pure substances— carbon dioxide, iron, oxygen, sugar.
(iv) Non-uniform mixtures — air, sand, brass, muddy water.
Ans: (iii) Pure substances— carbon dioxide, iron, oxygen, sugar.
- Correct option: Option (iii) iron and oxygen are elements (cannot be broken down), while carbon dioxide and sugar are compounds (made of elements in fixed ratios).
- Option (i) incorrectly includes water (compound) and air (mixture);
- Option (ii) lists minerals (compounds/elements), seawater and air (mixtures), but bronze is uniform;
- Option (iv) has air (uniform mixture) and brass (uniform alloy), not all non-uniform.
Q5. Iron reacts with moist air to form iron oxide, and magnesium burns in oxygen to form magnesium oxide. Classify all the substances involved in the above reactions as elements, compounds or mixtures, with justification.
Ans:
- Iron: Element (pure metal, cannot be broken down).
- Moist air: Mixture (air gases plus water vapor, components retain properties).
- Iron oxide: Compound (iron and oxygen combined chemically).
- Magnesium: Element (pure metal).
- Oxygen: Element (pure gas).
- Magnesium oxide: Compound (magnesium and oxygen in fixed ratio).
- Justification: Elements are simplest substances; compounds form from elements via chemical reactions with new properties; mixtures do not involve chemical bonding.
Q6. Classify the following as elements, compounds, or mixtures in Table 8.3.
Carbon dioxide, sand, seawater, magnesium oxide, muddy water, aluminium, gold, oxygen, rust, iron sulfide, glucose, air, water, fruit juice, nitrogen, sodium chloride, sulfur, hydrogen, baking soda. Identify pure substances amongst these and list them below
Identify pure substances amongst these and list them below
Ans: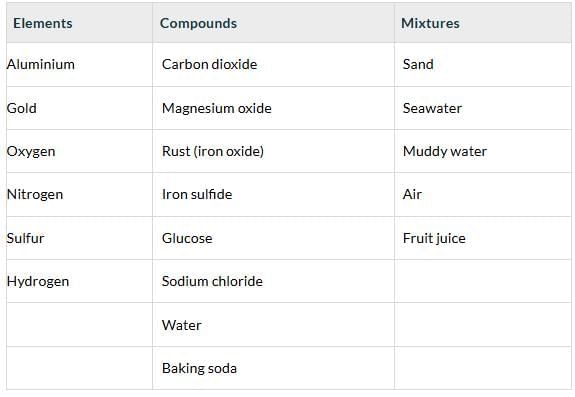
Pure substances: Carbon dioxide, magnesium oxide, rusty (iron oxide), iron sulfide, glucose, water, sodium chloride, baking soda, aluminium, gold, oxygen, nitrogen, sulfur, hydrogen. (These are either elements or compounds with uniform particles).
Q7. What new substance is formed when a mixture of iron filings and sulfur powder is heated, and how is it different from the original mixture? Also, write the word equation for the reaction.
Ans: Iron sulfide (a black compound) is formed. It differs as it has uniform texture, no magnetic attraction (unlike iron in the mixture), and reacts with hydrochloric acid to produce hydrogen sulfide (rotten egg odor), not hydrogen gas like the mixture. Word equation: Iron + Sulfur → Iron sulfide.
Q8. Is it possible for a substance to be classified as both an element and a compound? Explain why or why not.
Ans: No, because elements are simplest substances made of identical atoms and cannot be broken down, while compounds are made of two or more elements chemically combined in fixed ratios with different properties. A substance cannot be both simplest and composed of multiple elements.
Q9. How would our daily lives be changed if water were not a compound but a mixture of hydrogen and oxygen?
Ans: Water as a mixture would separate easily into flammable hydrogen and combustion-supporting oxygen, making it explosive and unusable for drinking, cooking, or extinguishing fires. It would lack stability, affecting life processes, weather, and ecosystems reliant on water's unique properties as a compound.
Q10. Analyse Fig. 8.24. Identify Gas A. Also, write the word equation of the chemical reaction.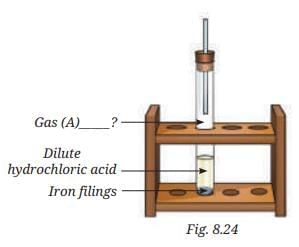
Ans: Gas A is hydrogen (produced when iron reacts with acid, burning with a pop sound). Word equation: Iron + Dilute hydrochloric acid → Iron chloride + Hydrogen.
Q11. Write the names of any two compounds made only from non-metals, and also mention two uses of each of them.
Ans:
Carbon dioxide: Used in fire extinguishers (absorbs heat, displaces oxygen) and aerated drinks (provides fizz).
Water: Used for hydration (essential for life) and cleaning (dissolves substances).
Q12. How can gold be classified as both a mineral and a metal?
Ans: Gold is a native mineral (pure element found naturally in rocks) and a metal (shiny, malleable, conductive element). It fits both as it's an element in pure form, not a compound, unlike most minerals.
Discover, design, and debate
Q1. Design and create comic strips from real-life examples to differentiate between elements, compounds, and mixtures with diagrams and illustrate their properties and uses.
Ans:
Comic Strip Idea:
Panel 1: Element (iron) – A superhero "Iron Atom" stands alone, unbreakable.
Panel 2: Compound (water) – Hydrogen and oxygen "team up" chemically to form water, extinguishing a fire (unlike their flammable selves). Panel 3: Mixture (air) – Gases like nitrogen and oxygen mix loosely, shown as friends hanging out without changing. Include diagrams of atoms/molecules and uses (e.g., iron in bridges, water for life, air for breathing).
Q2. Search for discoveries of some elements (such as phosphorus, sodium), compounds (such as penicillin) and mixtures (such as brass, bronze, stainless steel). Present your findings in the class.
Ans:
- Phosphorus: Discovered in 1669 by Hennig Brand from urine; used in matches and fertilizers.
- Sodium: Isolated in 1807 by Humphry Davy via electrolysis; used in soaps and streetlights.
- Penicillin: Discovered in 1928 by Alexander Fleming from mold; antibiotic compound saving lives from infections.
- Brass: Ancient mixture of copper and zinc; used in musical instruments for durability.
- Bronze: Early alloy of copper and tin; for statues and tools.
- Stainless steel: Modern mixture with iron, chromium; rust-resistant for utensils. Present as a slideshow with timelines.
Q3. Let us search: Read labels on items like detergents or snacks, and try to list the mixtures and compounds they contain.
Ans:
- Detergent label: Mixtures – surfactants with water; Compounds – sodium carbonate (cleaning agent), sodium sulfate (filler).
- Snacks (chips): Mixtures – potato with oil and spices; Compounds – sodium chloride (salt), citric acid (flavoring).
Q4. Work in groups: Each group will pretend to be in the role of either an element, a compound, or a mixture. Debate which category among them is the most important.
Ans: Debate Structure: Group 1 (Elements) argues they are building blocks (e.g., oxygen for life).
Group 2 (Compounds) claims innovation (e.g., water sustains ecosystems).
Group 3 (Mixtures) highlights versatility (e.g., air for breathing, alloys for tools). Conclude all are essential, as matter relies on their interplay.
|
136 videos|530 docs|57 tests
|
FAQs on NCERT Textbook Solutions: Nature of Matter: Elements, Compounds, and Mixtures - Science Class 8
| 1. What is the difference between elements, compounds, and mixtures? |  |
| 2. How can we separate mixtures into their components? |  |
| 3. What are some examples of compounds found in everyday life? |  |
| 4. Can elements exist in different forms? If so, what are they? |  |
| 5. Why is understanding the nature of matter important in science? |  |
















