NEET Exam > NEET Notes > Chemistry Class 12 > Mind Map: D and F - Block Elements
Mind Map: D and F - Block Elements | Chemistry Class 12 - NEET PDF Download
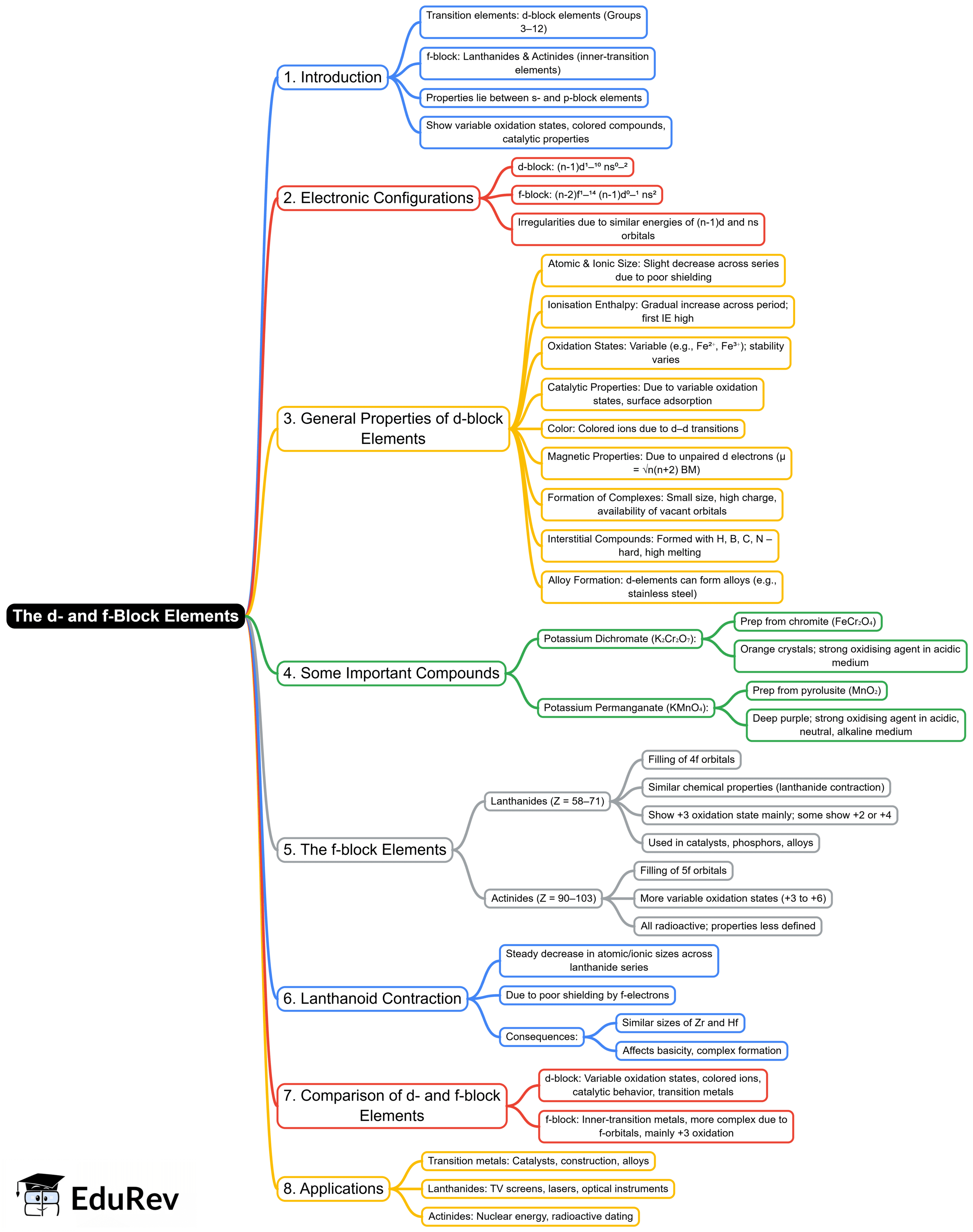
The document Mind Map: D and F - Block Elements | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|339 docs|78 tests
|
FAQs on Mind Map: D and F - Block Elements - Chemistry Class 12 - NEET
| 1. What are the key characteristics of D-block elements in the periodic table? |  |
Ans. D-block elements, also known as transition metals, are characterized by the presence of d-electrons in their penultimate shell. They typically have high melting and boiling points, exhibit variable oxidation states, and are good conductors of electricity and heat. Additionally, they form colored compounds and have complex ions due to their ability to undergo d-d transitions.
| 2. How do F-block elements differ from D-block elements in terms of electronic configuration? |  |
Ans. F-block elements consist of the lanthanides and actinides, where the f-orbitals are being filled. Their general electronic configuration is [Xe] 6s² 4f¹⁻¹⁴ for lanthanides and [Rn] 7s² 5f¹⁻¹⁴ for actinides. In contrast, D-block elements have their d-orbitals being filled, with a general configuration of [Noble gas] ns² (n-1)d¹⁻¹⁰. This fundamental difference in electronic configuration leads to distinct chemical properties and behaviors.
| 3. What roles do D and F-block elements play in industrial applications? |  |
Ans. D-block elements are widely used in various industries due to their catalytic properties, strength, and conductivity. For example, iron is crucial in construction, while nickel is essential in battery production. F-block elements, particularly those from the actinide series like uranium and plutonium, are vital in nuclear energy and weaponry. Lanthanides are used in manufacturing strong permanent magnets and phosphors for LED lights.
| 4. Why are D and F-block elements often referred to as heavy metals? |  |
Ans. D and F-block elements are often referred to as heavy metals because they have higher atomic masses and densities compared to other elements. These properties contribute to their strength and durability, making them suitable for various applications, such as construction materials and specialized equipment. However, the term "heavy metals" can also imply toxicological properties, as many of these elements can be harmful in excessive amounts.
| 5. What are some common oxidation states of D-block and F-block elements? |  |
Ans. D-block elements exhibit a range of oxidation states, commonly from +1 to +7, depending on the element. For instance, manganese can exhibit oxidation states from +2 to +7. F-block elements, particularly lanthanides, typically show a +3 oxidation state, while actinides can have multiple oxidation states, including +3, +4, and +5. The variability in oxidation states is due to the involvement of d and f electrons in bonding and ion formation.
Related Searches
















