NEET Exam > NEET Notes > Chemistry Class 12 > Infographic: p-Block Elements
Infographic: p-Block Elements | Chemistry Class 12 - NEET PDF Download
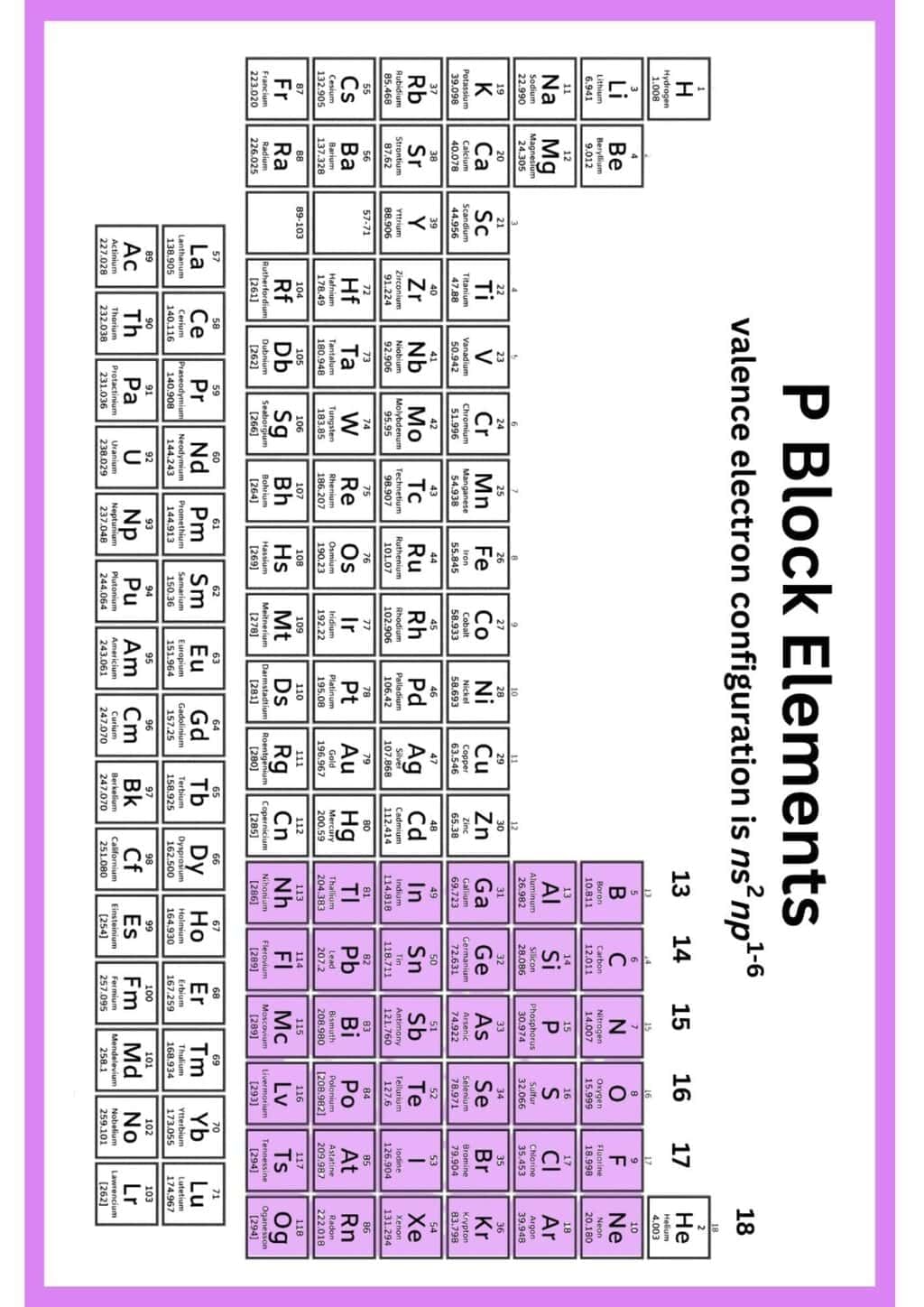
The document Infographic: p-Block Elements | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|339 docs|78 tests
|
FAQs on Infographic: p-Block Elements - Chemistry Class 12 - NEET
| $1. What are p-block elements and why are they important in chemistry? |  |
Ans. p-block elements are the set of elements in groups 13 to 18 of the periodic table. They are characterized by the filling of p orbitals in their electronic configuration. These elements include metals, metalloids, and non-metals, and they play crucial roles in various chemical reactions, biological processes, and industrial applications. Their diverse properties and compounds make them essential for understanding chemical behavior and interactions.
| $2. What are the common oxidation states of p-block elements? |  |
Ans. The common oxidation states of p-block elements vary widely. For instance, Group 13 elements typically exhibit +1 and +3 oxidation states, Group 14 elements can show +4 and +2, while Group 15 elements often display -3, +3, and +5 states. Group 16 elements usually have -2, +4, and +6 oxidation states, and Group 17 elements are commonly found in -1 and +1 states. These oxidation states are fundamental for predicting the reactivity and types of compounds formed by these elements.
| $3. How do p-block elements differ from s-block elements? |  |
Ans. p-block elements differ from s-block elements primarily in their electronic configurations and properties. S-block elements (groups 1 and 2) have their outermost electrons in s orbitals, making them highly reactive metals. In contrast, p-block elements have their outermost electrons in p orbitals, leading to a wider variety of chemical behaviors, including metals, metalloids, and non-metals. This diversity results in varied physical and chemical properties, as well as applications in different fields.
| $4. What are some important compounds formed by p-block elements? |  |
Ans. P-block elements form a variety of important compounds, including but not limited to:
- Aluminum oxide (Al₂O₃), used in ceramics and abrasives.
- Silicon dioxide (SiO₂), a primary component of glass and sand.
- Phosphoric acid (H₃PO₄), utilized in fertilizers and food flavoring.
- Nitrogen oxides (NO and NO₂), significant in environmental chemistry and pollution.
These compounds are crucial in numerous industries and everyday applications.
| $5. How do p-block elements contribute to biological systems? |  |
Ans. P-block elements play vital roles in biological systems. For instance, nitrogen (from Group 15) is essential for the formation of amino acids and nucleic acids. Phosphorus (also from Group 15) is a key component of ATP, DNA, and RNA, which are critical for energy transfer and genetic information. Additionally, various metalloids and metals from the p-block, such as boron and aluminum, are involved in enzyme activity and metabolic processes, highlighting their significance in life sciences.
Related Searches





















