Revision Notes: d- and f-Block Elements | Chemistry Class 12 - NEET PDF Download
d- and f-Block Elements
The d- and f-block elements, known as transition and inner transition elements, are located in the central region of the periodic table. This chapter covers their electronic configurations, properties, and applications, vital for NEET preparation.
Position in Periodic Table
- d-Block: Groups 3 to 12 (transition elements).
- f-Block: Lanthanoids (4f series) and Actinoids (5f series; inner transition elements).
d-Block Elements (Transition Elements)
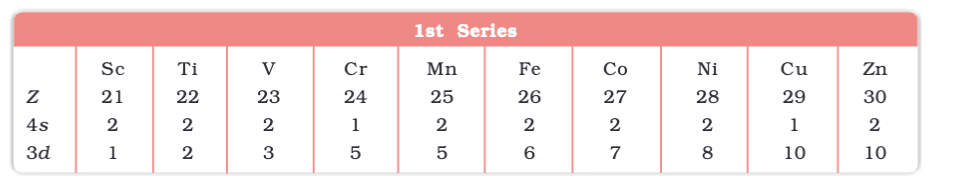
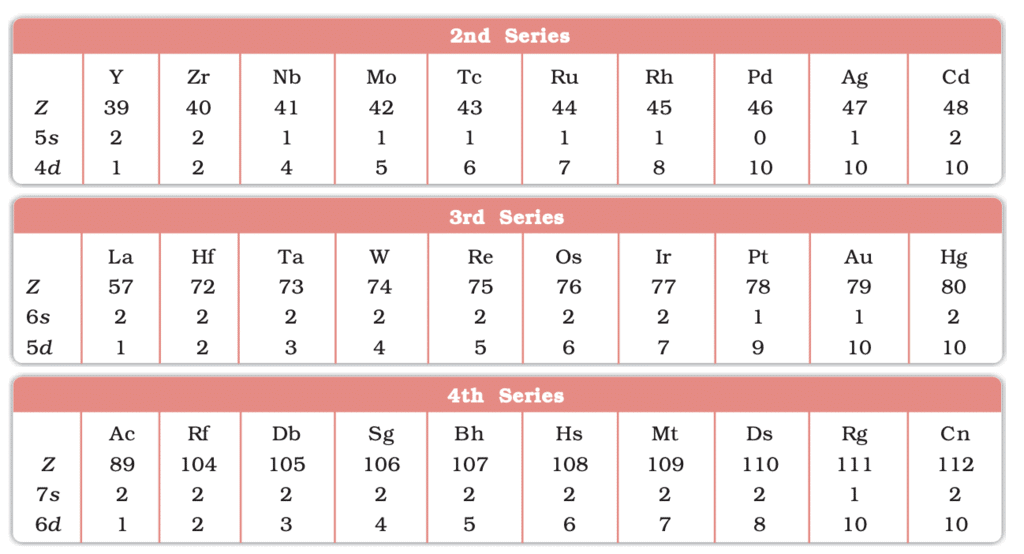
Electronic Configurations of outer orbitals of the Transition Elements(ground state)
General Characteristics
- Electronic Configuration: (n-1)d¹⁻¹⁰ ns¹⁻²; d-orbitals partially filled in ions.
- Properties:
- High melting/boiling points (strong metallic bonding).
- Variable oxidation states (e.g., Mn: +2 to +7).
- Colored ions (d-d electron transitions).
- Catalytic activity (e.g., Pt in catalytic converters).
- Magnetic behavior: Paramagnetic (unpaired electrons).
- Examples: Fe, Cu, Ni, Zn.
Series
- 3d: Sc (21) to Zn (30).
- 4d: Y (39) to Cd (48).
- 5d: La (57), Hf (72) to Hg (80).
- 6d: Ac (89), Rf (104) to Cn (112).
Trends in Properties
- Atomic/Ionic Radii: Decrease across period, then stabilize (d-orbital shielding).
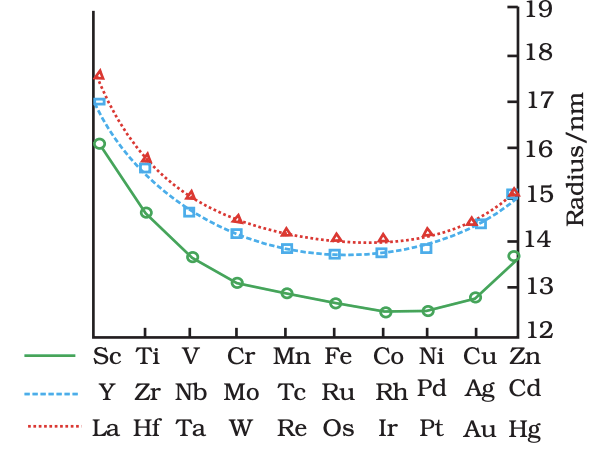 Trends in atomic radii oftransition elements
Trends in atomic radii oftransition elements - Ionization Enthalpy: Increases generally; exceptions (e.g., Cr, Cu due to stability).
- Oxidation States: Peak in middle (e.g., Mn: +7), decrease towards ends.
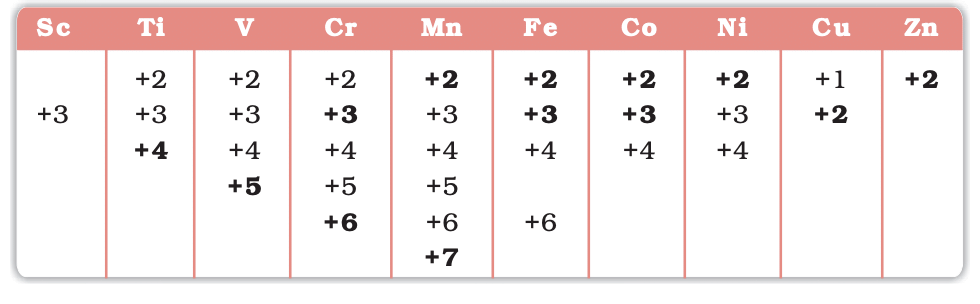 Oxidation States of the first row Transition Metal(the most common ones are in bold types)
Oxidation States of the first row Transition Metal(the most common ones are in bold types)
f-Block Elements (Inner Transition Elements)
Lanthanoids
- Position: Ce (58) to Lu (71); 4f¹⁻¹⁴.
- Electronic Configuration: [Xe] 4f¹⁻¹⁴ 5d⁰⁻¹ 6s².
- Properties:
- Silvery-white, soft metals.
- Dominant oxidation state: +3 (some +2, +4).
- Lanthanoid contraction: Size decreases due to poor 4f shielding.
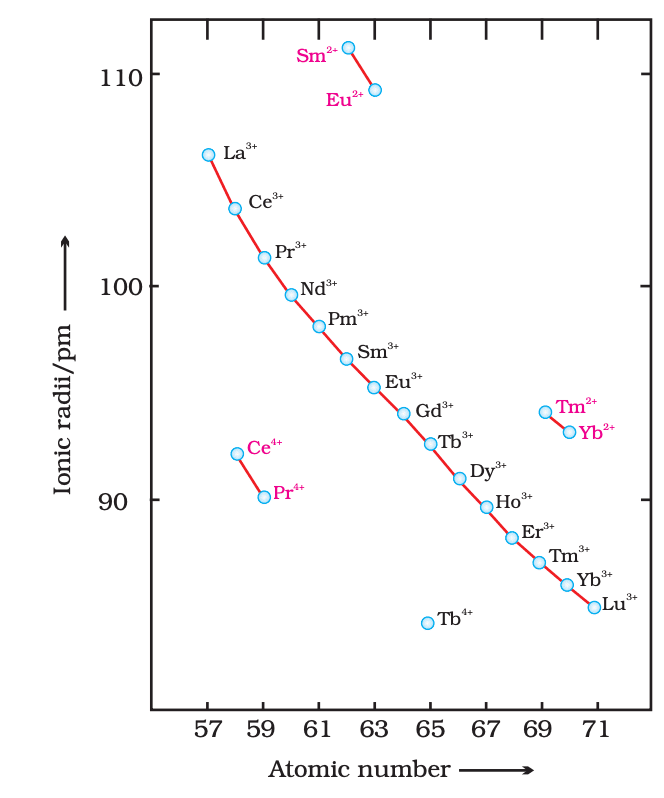
- Paramagnetic (unpaired 4f electrons; exceptions: La³⁺, Lu³⁺).
- Examples: Ce, Pr, Eu.
Actinoids
- Position: Th (90) to Lr (103); 5f¹⁻¹⁴.
- Electronic Configuration: [Rn] 5f¹⁻¹⁴ 6d⁰⁻¹ 7s².
- Properties:
- Radioactive; most are synthetic (beyond U).
- Multiple oxidation states (+3 to +6; e.g., U: +6).
- Actinoid contraction: Similar to lanthanoids.
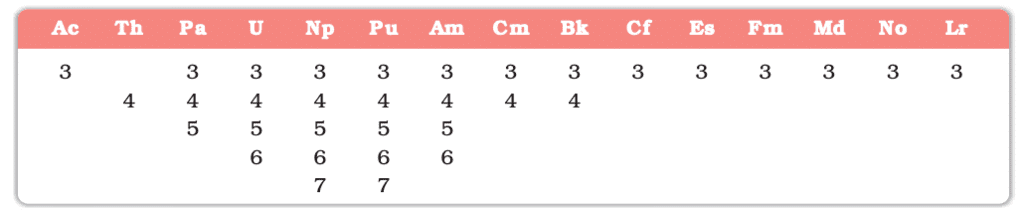
- Highly reactive and dense.
- Examples: Th, U, Np.
Important Compounds
Potassium Dichromate (K₂Cr₂O₇)
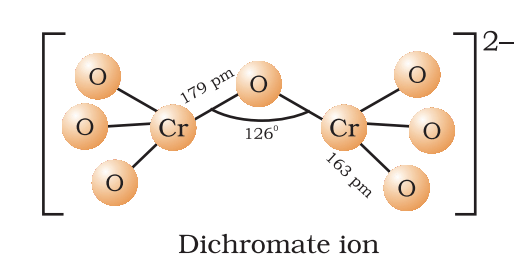
- Preparation: 4NaCl + K₂Cr₂O₇ + 2H₂SO₄ → K₂Cr₂O₇ + 2Na₂SO₄ + 2HCl.
- Properties: Orange-red crystals; oxidizes (Cr⁶⁺ → Cr³⁺).
- Uses: Oxidizing agent, chrome tanning, alcohol breath test.
Potassium Permanganate (KMnO₄)
- Preparation: 2MnO₂ + 4KOH + O₂ → 2K₂MnO₄ + 2H₂O; then K₂MnO₄ + Cl₂ → 2KMnO₄.
- Properties: Purple crystals; strong oxidizer (Mn⁷⁺ → Mn²⁺).
- Uses: Disinfectant, bleaching, titrations.
Chemical Properties
Oxidation States
- d-Block: Vary widely (e.g., Fe: +2, +3; Cr: +3, +6).
- f-Block: Lanthanoids (+3); Actinoids (+3 to +6).
Color and Magnetism
- Color: d-d transitions (e.g., CuSO₄: blue); f-block less intense.
- Magnetism: Paramagnetic (unpaired e⁻); Diamagnetic (all paired).
Catalytic Properties
- Due to variable oxidation states (e.g., Fe in ammonia synthesis).
Applications
d-Block
- Alloys (e.g., brass: Cu + Zn), catalysts (e.g., Ni in hydrogenation).
f-Block
- Lanthanoids: Glass polishing (Ce), magnets (Nd).
- Actinoids: Nuclear reactors (U, Pu).
Points to Remember
- d-Block: Groups 3–12; (n-1)d¹⁻¹⁰ ns¹⁻²; colored, catalytic.
- f-Block: 4f (lanthanoids), 5f (actinoids); +3 dominant, radioactive (actinoids).
- Contraction: Lanthanoid (4f), Actinoid (5f) due to poor shielding.
- Compounds: K₂Cr₂O₇ (orange, Cr⁶⁺), KMnO₄ (purple, Mn⁷⁺).
- Properties: Variable oxidation, magnetism, catalysis.
Additional Key Concepts
- Zn (d¹⁰) not a true transition element (fixed +2 state).
- Max oxidation: Cr (+6), Mn (+7) in K₂Cr₂O₇, KMnO₄.
- Actinoids beyond U are man-made (transuranics).
- Color intensity: d-block > f-block (f-f transitions weak).
- Shielding order: s > p > d > f.
General Points and Errors to Be Noted
- Do not confuse transition (d) with inner transition (f) elements.
- Memorize key oxidation states and compound colors.
- Avoid mixing K₂Cr₂O₇ (Cr) with KMnO₄ (Mn).
- Understand contraction impacts atomic radii (e.g., Zr ≈ Hf).
Example Problems
Example 1
Question: The purple color of KMnO₄ is due to:
- Cr⁶⁺
- Mn⁷⁺
- Fe³⁺
- Cu²⁺
Answer: b) Mn⁷⁺
Solution: KMnO₄ contains Mn⁷⁺, responsible for its purple color due to charge transfer.
Example 2
Question: Which element exhibits the most oxidation states?
- Fe
- Cr
- Mn
- Zn
Answer: c) Mn
Solution: Mn shows +2 to +7 (e.g., MnO₄⁻), more than Fe (+2, +3), Cr (+2, +3, +6), or Zn (+2).
Example 3
Question: Lanthanoid contraction affects:
- 3d series
- 4d series
- 5d series
- 6d series
Answer: c) 5d series
Solution: Lanthanoid contraction (4f) reduces size of 5d elements (e.g., Hf ≈ Zr).
|
75 videos|338 docs|78 tests
|
FAQs on Revision Notes: d- and f-Block Elements - Chemistry Class 12 - NEET
| 1. What are the main characteristics of d-block elements? |  |
| 2. Why are f-block elements called inner transition metals? |  |
| 3. How do the oxidation states of d-block elements vary? |  |
| 4. What is the significance of coordination compounds in d-block elements? |  |
| 5. How do f-block elements differ from d-block elements in terms of reactivity? |  |





















