EmSAT Achieve Exam > EmSAT Achieve Notes > Chemistry for EmSAT Achieve > Mind Map: Gas laws
Mind Map: Gas laws | Chemistry for EmSAT Achieve PDF Download
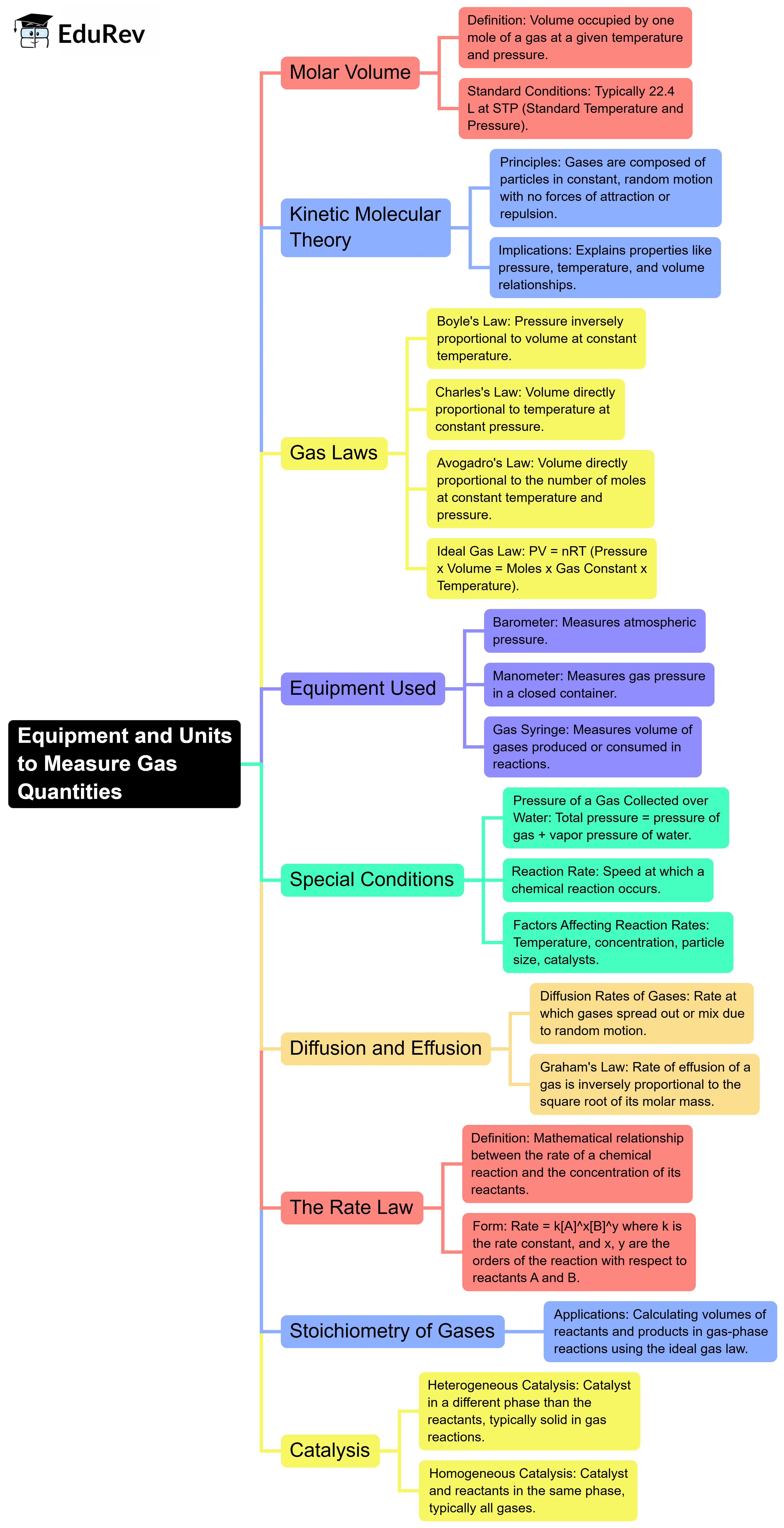
The document Mind Map: Gas laws | Chemistry for EmSAT Achieve is a part of the EmSAT Achieve Course Chemistry for EmSAT Achieve.
All you need of EmSAT Achieve at this link: EmSAT Achieve
|
198 videos|319 docs|164 tests
|
FAQs on Mind Map: Gas laws - Chemistry for EmSAT Achieve
| 1. What are the main gas laws and how do they relate to each other? |  |
Ans. The main gas laws include Boyle's Law, Charles's Law, Avogadro's Law, and the Ideal Gas Law. Boyle's Law states that the pressure of a gas is inversely proportional to its volume at constant temperature (P₁V₁ = P₂V₂). Charles's Law states that the volume of a gas is directly proportional to its temperature at constant pressure (V₁/T₁ = V₂/T₂). Avogadro's Law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules (V₁/n₁ = V₂/n₂). The Ideal Gas Law combines these relationships into one equation: PV = nRT, where R is the gas constant. These laws help us understand the behavior of gases under various conditions.
| 2. How do temperature and pressure affect gas volume according to the gas laws? |  |
Ans. According to Charles's Law, as the temperature of a gas increases, its volume also increases if the pressure is held constant. Conversely, Boyle's Law indicates that if the pressure of a gas increases, its volume decreases, provided the temperature remains constant. Together, these laws show that temperature and pressure have a significant impact on gas volume, illustrating the dynamic nature of gases in different environments.
| 3. What is the Ideal Gas Law, and what assumptions does it make? |  |
Ans. The Ideal Gas Law is represented by the equation PV = nRT. It relates the pressure (P), volume (V), number of moles (n), the ideal gas constant (R), and temperature (T) of an ideal gas. The assumptions of the Ideal Gas Law include that gas particles do not attract or repel each other, the volume of gas particles is negligible compared to the volume of the container, and that collisions between gas particles are perfectly elastic. These assumptions simplify calculations and provide a good approximation for many gases under standard conditions.
| 4. How do real gases differ from ideal gases, and when do these differences become significant? |  |
Ans. Real gases differ from ideal gases in that they can experience intermolecular forces and occupy a finite volume. Under high pressures and low temperatures, these differences become significant, as the assumptions of the Ideal Gas Law no longer hold true. Real gases deviate from ideal behavior at these extremes due to interactions between gas particles and the physical space they occupy, leading to corrections in calculations.
| 5. How can gas laws be applied in real-life situations? |  |
Ans. Gas laws have various applications in real-life situations, such as calculating the behavior of gases in weather balloons, determining the pressure changes in car tires with temperature variations, and understanding how breathing works in respiratory systems. For instance, Boyle's Law explains how a syringe functions when drawing in or expelling air, while Charles's Law is relevant in understanding how hot air balloons rise as the air inside them heats up, causing an increase in volume.
Related Searches















