Unit Test: Nature of Matter: Elements, Compounds, and Mixtures | Science Curiosity Class 8 - New NCERT PDF Download
Time: 1 hour
M.M. 30
Attempt all questions.
- Question numbers 1 to 5 carry 1 mark each.
- Question numbers 6 to 8 carry 2 marks each.
- Question numbers 9 to 11 carry 3 marks each.
- Question numbers 12 & 13 carry 5 marks each.
- 1-mark questions include MCQs.
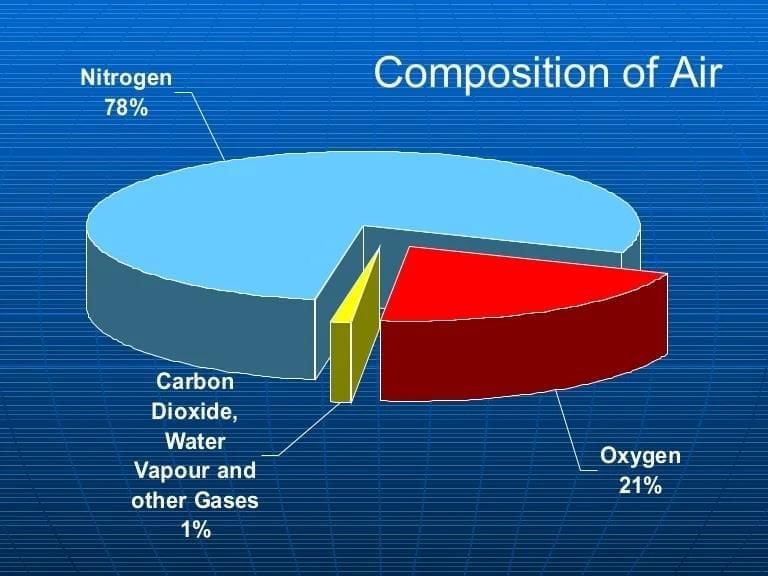
Q1: Air is best described as (1 Mark)
(i) an element
(ii) a compound
(iii) a uniform (homogeneous) mixture
(iv) a non-uniform (heterogeneous) mixture
Q2: A mixture is formed when two or more substances are combined such that (1 Mark)
(i) they chemically react to form a new substance
(ii) each component retains its properties and can be separated by physical means
(iii) the components necessarily have fixed ratios
(iv) the components are always visible
Q3: Which of the following is a pure substance in the scientific sense? (1 Mark)
(i) Seawater
(ii) Air
(iii) Oxygen gas
(iv) Milk
Q4: When water is decomposed by passing electricity, the products are (1 Mark)
(i) hydrogen and oxygen
(ii) hydrogen only
(iii) oxygen only
(iv) hydrogen and carbon dioxide
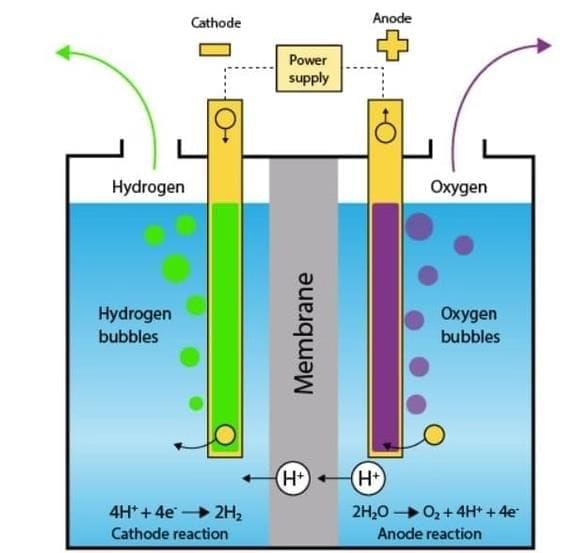 Electrolysis of Water
Electrolysis of Water
Q5: Which statement about compounds is correct? (1 Mark)
(i) Components are present in any proportion and retain their properties.
(ii) Components are present in fixed ratios and the compound has new properties.
(iii) Components can be separated by filtration and evaporation only.
(iv) Compounds are always gases.
Q6: Give one everyday example each of a uniform mixture and a non-uniform mixture; explain why. (2 Marks)
Q7: “Pure” in everyday language vs “pure” in science—differentiate with one example. (2 Marks)
Q8: Classify stainless steel and brass; justify your answer. (2 Marks)
Q9: In the “lime water” activity, lime water turns milky when exposed to air. Explain the observation and write the word equation. (3 Marks)
Q10: Consider the mixture of iron filings and sulfur (Sample A) and the heated product (Sample B). Describe two tests that distinguish them and the inferences. (3 Marks)
Q11: A student says, “Sugar is an element because it looks uniform.” Correct this misconception using evidence from heating sugar in a test tube. (3 Marks)
Q12: (a) Define element, compound, and mixture with one example each.
(b) Why can’t the elements of a compound be separated by physical methods, whereas mixture components can?
(c) For water, state the fixed ratio and contrast its properties with hydrogen and oxygen. (5 Marks)
Q13: Extended reasoning and classification.
(a) Explain why air quality measurements report “particulate matter” although air is a uniform mixture.
(b) Classify each as element, compound, or mixture, with brief justification: oxygen, sodium chloride, seawater, iron sulfide, muddy water, glucose, brass, nitrogen, baking soda, sand.
(c) Using Below Figure: iron + dilute HCl → Gas A), identify Gas A and write the word equation. (5 Marks)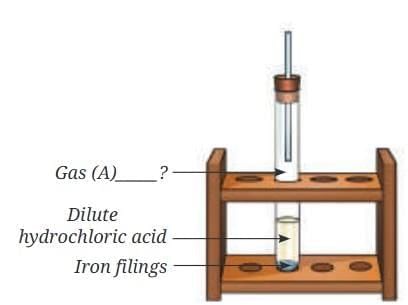
|
54 videos|234 docs|13 tests
|
FAQs on Unit Test: Nature of Matter: Elements, Compounds, and Mixtures - Science Curiosity Class 8 - New NCERT
| 1. What is the difference between elements, compounds, and mixtures? |  |
| 2. How can we separate mixtures into their components? |  |
| 3. Can you give examples of everyday mixtures and their components? |  |
| 4. What are some common compounds and their uses in everyday life? |  |
| 5. Why is it important to understand the differences between elements, compounds, and mixtures? |  |





















