Class 7 Exam > Class 7 Notes > Science Olympiad Class 7 > Mind Map: The World of Metals and Non-metals
Class 7 Science Chapter 4 Mindmap - The World of Metals and Non-metals
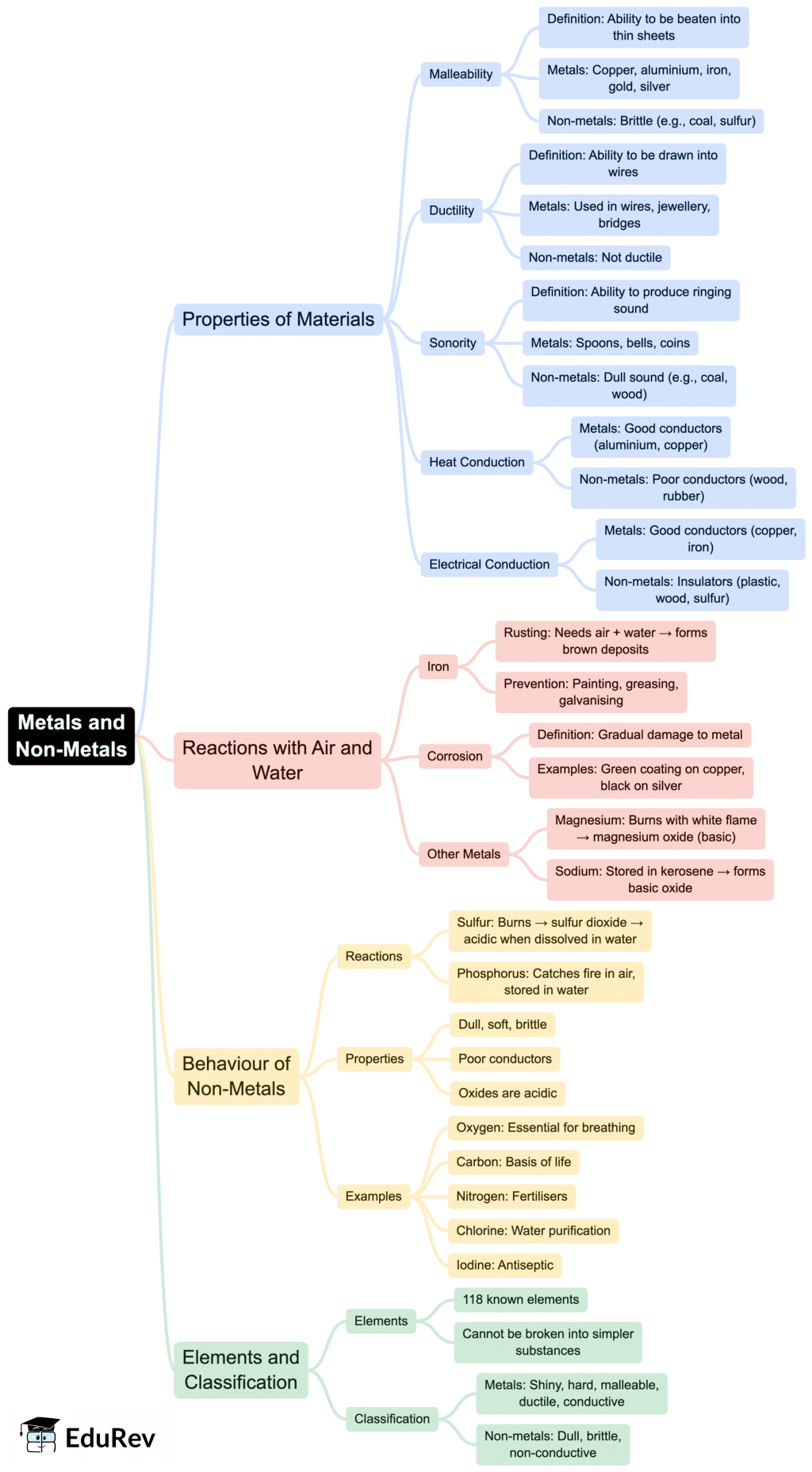
The document Class 7 Science Chapter 4 Mindmap - The World of Metals and Non-metals is a part of the Class 7 Course Science Olympiad Class 7.
All you need of Class 7 at this link: Class 7
|
33 videos|131 docs|54 tests
|
FAQs on Class 7 Science Chapter 4 Mindmap - The World of Metals and Non-metals
| 1. What are the main differences between metals and non-metals? |  |
Ans. Metals are typically shiny, good conductors of heat and electricity, and can be easily shaped or molded. They are usually solid at room temperature (except mercury). Non-metals, on the other hand, are often dull, poor conductors, and can exist in solid, liquid, or gaseous states. Non-metals tend to be brittle in solid form and do not have the same malleability or ductility as metals.
| 2. Why are metals considered good conductors of electricity? |  |
Ans. Metals have free-moving electrons that can carry electrical current. When an electric field is applied to a metal, these electrons move easily through the lattice structure of the metal, allowing electricity to flow. This property makes metals like copper and aluminum ideal for electrical wiring.
| 3. Can you give examples of common metals and non-metals? |  |
Ans. Common metals include iron, copper, aluminum, and gold. These are often used in construction, electronics, and jewelry. Common non-metals include sulfur, oxygen, nitrogen, and carbon. These are found in various compounds and are essential for life, such as oxygen for respiration and carbon for organic molecules.
| 4. What are some everyday uses of non-metals? |  |
Ans. Non-metals have various everyday applications. For instance, oxygen is crucial for breathing and combustion, nitrogen is used in fertilizers, and carbon is found in fuels and organic compounds. Other non-metals like chlorine are used in disinfectants, and phosphorus is essential in fertilizers.
| 5. How do metals and non-metals react chemically? |  |
Ans. Metals tend to lose electrons during chemical reactions, forming positive ions (cations), while non-metals tend to gain or share electrons, forming negative ions (anions) or covalent bonds. This difference in behavior leads to the formation of ionic and covalent compounds, respectively. For example, sodium (a metal) reacts with chlorine (a non-metal) to form sodium chloride (table salt).
Related Searches
















