JEE Exam > JEE Notes > Chemistry for JEE Main & Advanced > Mind Map: Amines
Mind Map: Amines | Chemistry for JEE Main & Advanced PDF Download
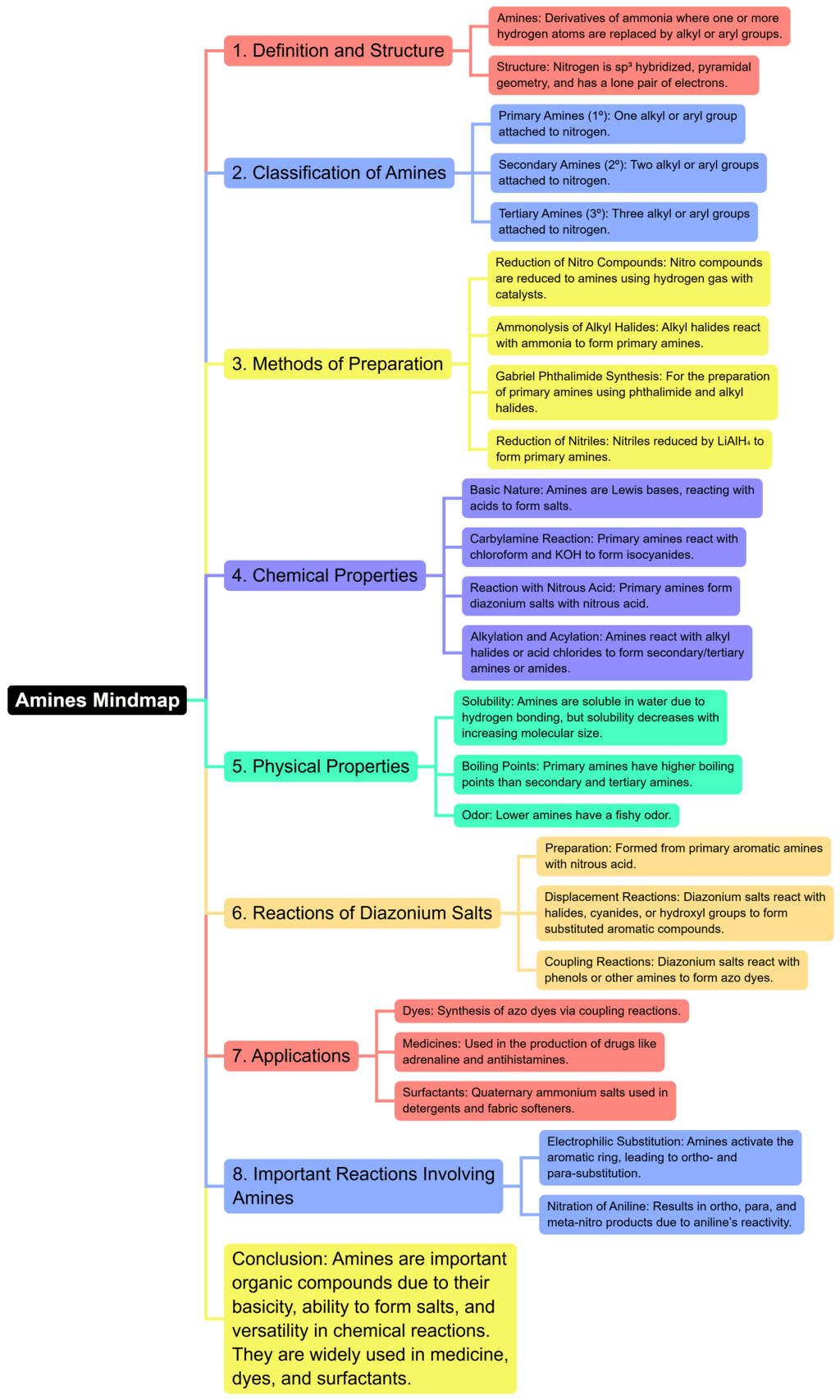
The document Mind Map: Amines | Chemistry for JEE Main & Advanced is a part of the JEE Course Chemistry for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
361 videos|822 docs|301 tests
|
FAQs on Mind Map: Amines - Chemistry for JEE Main & Advanced
| 1. What are amines and how are they classified? |  |
Ans. Amines are organic compounds that contain a nitrogen atom bonded to hydrogen atoms and/or carbon atoms. They are classified based on the number of carbon-containing groups attached to the nitrogen. Primary amines have one carbon group (R-NH₂), secondary amines have two (R₂NH), and tertiary amines have three (R₃N).
| 2. What is the basicity of amines and how does it compare to ammonia? |  |
Ans. Amines are basic due to the presence of a lone pair of electrons on the nitrogen atom, which can accept protons (H⁺). The basicity of amines is generally higher than that of ammonia because the presence of alkyl groups increases the electron density on the nitrogen atom, enhancing its ability to donate the lone pair to protons.
| 3. Describe the methods for synthesizing amines. |  |
Ans. Amines can be synthesized through several methods, including the reduction of nitro compounds, alkylation of ammonia or amines, and the reductive amination of carbonyl compounds. Common reducing agents for nitro compounds include lithium aluminum hydride (LiAlH₄) and hydrogen in the presence of catalysts, which convert nitro groups to amine groups.
| 4. What are the physical properties of amines? |  |
Ans. Amines generally have higher boiling points compared to hydrocarbons of similar molecular weight due to their ability to form hydrogen bonds. They are typically polar and soluble in water, particularly lower molecular weight amines, due to hydrogen bonding with water molecules. However, the solubility decreases as the alkyl chain length increases.
| 5. How do amines react with acids and what is the significance of this reaction? |  |
Ans. Amines react with acids to form ammonium salts. This reaction is significant because it demonstrates the basic nature of amines and their ability to neutralize acids. The formation of ammonium salts is also important in pharmaceutical applications, as it can influence the solubility and bioavailability of drugs containing amine functional groups.
Related Searches
















