JEE Exam > JEE Notes > Chemistry for JEE Main & Advanced > Mind Map: Alcohols, Phenols and Ethers
Mind Map: Alcohols, Phenols and Ethers | Chemistry for JEE Main & Advanced PDF Download
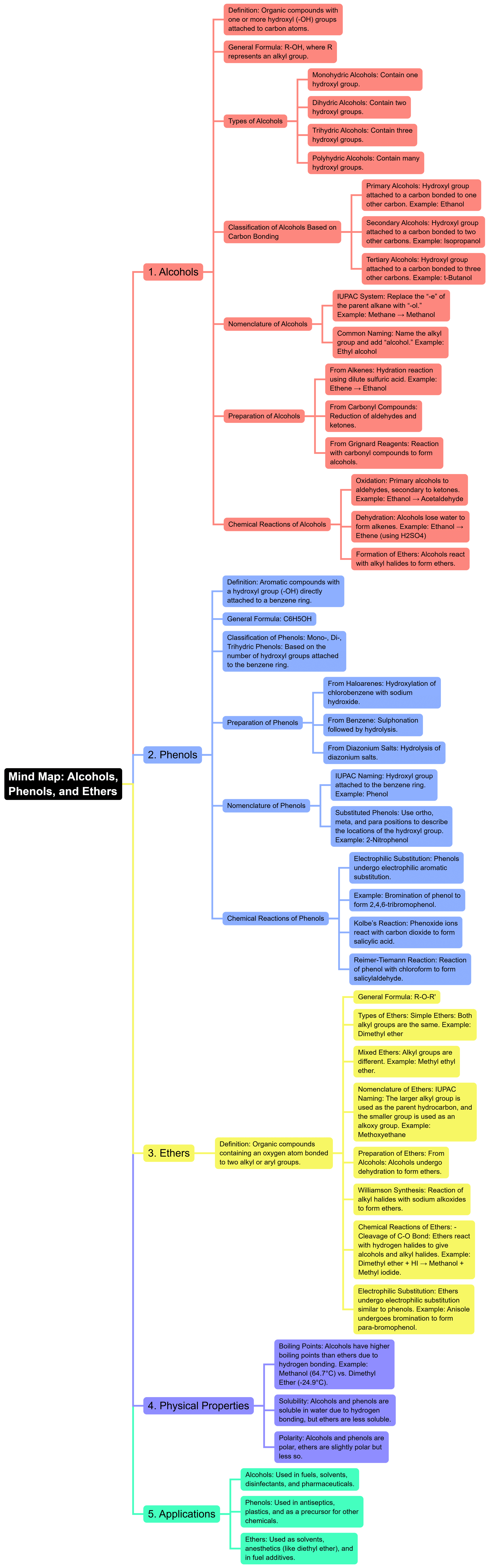
The document Mind Map: Alcohols, Phenols and Ethers | Chemistry for JEE Main & Advanced is a part of the JEE Course Chemistry for JEE Main & Advanced.
All you need of JEE at this link: JEE
|
361 videos|822 docs|301 tests
|
FAQs on Mind Map: Alcohols, Phenols and Ethers - Chemistry for JEE Main & Advanced
| 1. What are the main differences between alcohols, phenols, and ethers? |  |
Ans. Alcohols are organic compounds characterized by the presence of one or more hydroxyl (-OH) groups attached to a carbon atom. Phenols are similar but contain a hydroxyl group directly bonded to an aromatic ring. Ethers, on the other hand, have an oxygen atom bonded to two alkyl or aryl groups, represented by the general formula R-O-R'. The key difference lies in their functional groups and structure, which leads to distinct chemical properties and reactivity.
| 2. How do you classify alcohols based on their structure? |  |
Ans. Alcohols can be classified into three categories based on the carbon atom to which the hydroxyl group is attached: primary (1°) alcohols have the -OH group attached to a carbon atom bonded to one other carbon, secondary (2°) alcohols have it attached to a carbon bonded to two other carbons, and tertiary (3°) alcohols have it attached to a carbon bonded to three other carbons. This classification is important for understanding their reactivity and oxidation behavior.
| 3. What are the common methods for preparing ethers? |  |
Ans. Ethers can be prepared through several methods, including the dehydration of alcohols, where two alcohol molecules react to form an ether and water, often in the presence of an acid catalyst. Another method is the Williamson ether synthesis, which involves the reaction of an alkoxide ion with a primary alkyl halide. These methods allow for the efficient synthesis of various ethers in the laboratory.
| 4. What is the significance of phenols in organic chemistry? |  |
Ans. Phenols are significant in organic chemistry due to their unique properties, such as acidity and the ability to form hydrogen bonds. They serve as important intermediates in the synthesis of a wide range of compounds, including pharmaceuticals, dyes, and antioxidants. The presence of the hydroxyl group on an aromatic ring also influences the reactivity and chemical behavior of phenols compared to aliphatic alcohols.
| 5. How do the physical properties of alcohols, phenols, and ethers differ? |  |
Ans. Alcohols and phenols generally have higher boiling points than ethers of similar molecular weight due to the presence of hydrogen bonding in alcohols and phenols. Ethers, while capable of dipole-dipole interactions, lack hydrogen bonding, resulting in lower boiling points. Additionally, alcohols and phenols tend to be more soluble in water due to their ability to form hydrogen bonds, whereas ethers have limited solubility depending on their structure.
Related Searches
















