Class 9 Exam > Class 9 Notes > Science Class 9 > Infographic: Nature of Matter: Elements, Compounds, and Mixtures
Infographic: Nature of Matter: Elements, Compounds, and Mixtures | Science Class 9 PDF Download
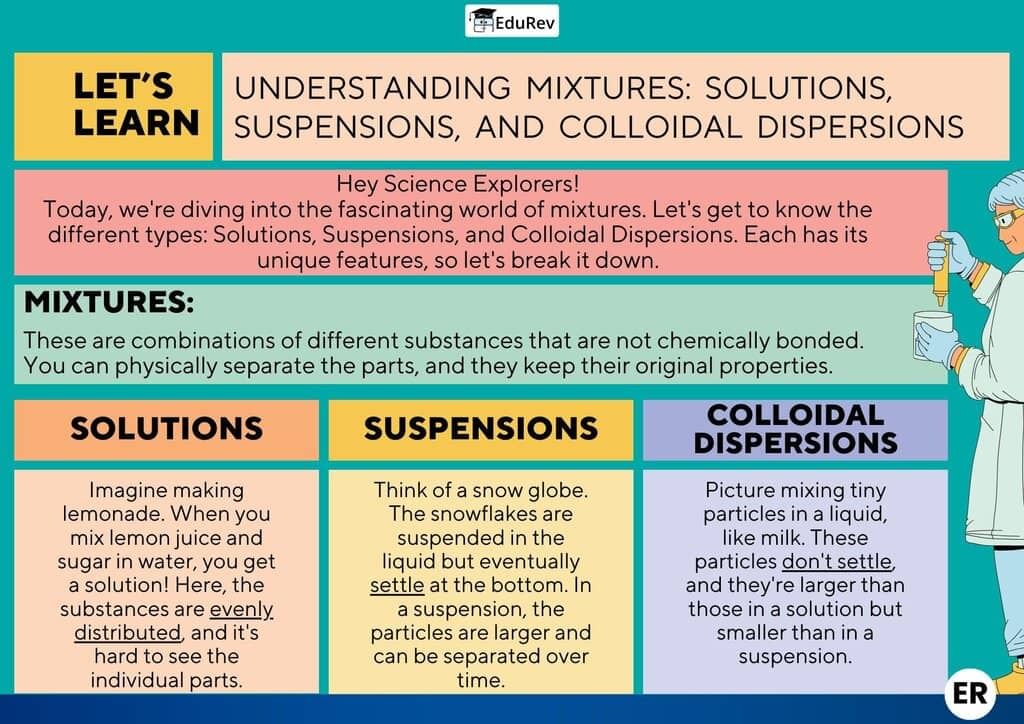
The document Infographic: Nature of Matter: Elements, Compounds, and Mixtures | Science Class 9 is a part of the Class 9 Course Science Class 9.
All you need of Class 9 at this link: Class 9
|
84 videos|541 docs|60 tests
|
FAQs on Infographic: Nature of Matter: Elements, Compounds, and Mixtures - Science Class 9
| 1. What is meant by a pure substance in the context of matter? |  |
Ans. A pure substance is a material that has a uniform and definite composition. It consists of only one type of particle, which can be either an element or a compound. For example, pure water (H₂O) is a compound made up of hydrogen and oxygen in a fixed ratio, while pure gold (Au) is an element that contains only gold atoms.
| 2. How can we differentiate between pure substances and mixtures? |  |
Ans. Pure substances have a consistent composition and distinct properties, while mixtures contain two or more different substances that retain their individual properties. Mixtures can be homogeneous (uniform composition, like saltwater) or heterogeneous (distinct components, like a salad). The separation of components in a mixture can often be achieved through physical methods, whereas pure substances cannot be separated into other materials by physical means.
| 3. What are the methods used to separate mixtures into their components? |  |
Ans. Common methods to separate mixtures include filtration, evaporation, distillation, and chromatography. Filtration is used to separate solids from liquids, evaporation removes a solvent from a solution, distillation separates components based on boiling points, and chromatography separates substances based on their movement through a medium.
| 4. Can you give examples of pure substances and mixtures found in everyday life? |  |
Ans. Yes, examples of pure substances include distilled water, table salt (NaCl), and oxygen gas (O₂). On the other hand, mixtures include air (a mixture of gases), salad (a mixture of various vegetables), and seawater (a mixture of water, salts, and other substances). These examples illustrate how pure substances and mixtures are common in our daily environment.
| 5. Why is it important to understand the concept of pure substances and mixtures in science? |  |
Ans. Understanding pure substances and mixtures is fundamental in science because it helps in the identification and classification of materials. This knowledge is crucial in various fields, including chemistry, biology, and environmental science, as it influences reactions, properties, and behaviors of different substances. Recognizing the difference aids in effective experimentation, analysis, and application of scientific principles in real-world situations.
Related Searches
















