|
A mineral is a naturally occurring chemical substance in which metal exists either in its free state or in a combined state. |
Card: 2 / 46 |
|
Gangue refers to the impurities associated with the ore that are not economically extracted. |
Card: 4 / 46 |
|
Carbonate vs Nitrate Ores Explained
|
Card: 6 / 46 |
|
Native ores are unreactive metals.
|
Card: 8 / 46 |
 The first step is pulverization, which involves crushing the ore to a powdered state. |
Card: 10 / 46 |
|
Concentration removes gangue from ore.
|
Card: 12 / 46 |
|
Fill in the blank: Froth flotation process is primarily used for the concentration of ___ ores. |
Card: 13 / 46 |
|
Frothers stabilize froth in flotation.
|
Card: 16 / 46 |
|
True or False: In the leaching process, the metallic ore is dissolved in a solvent while impurities remain insoluble. |
Card: 17 / 46 |
|
Calcination is a heating process.
|
Card: 20 / 46 |
|
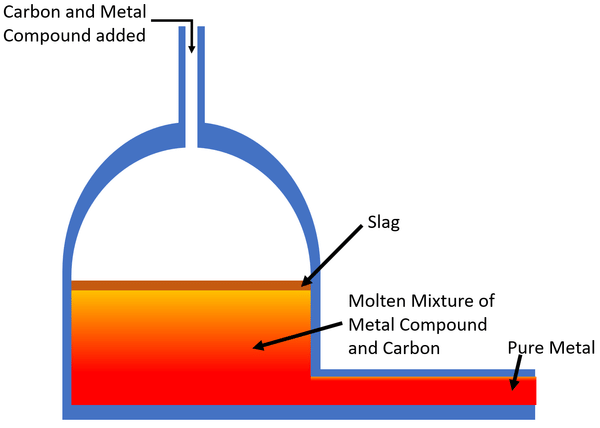
Reduction of metal oxides is key.
|
Card: 22 / 46 |
|
In the context of metallurgy, what is the significance of the Ellingham diagram? |
Card: 23 / 46 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Ellingham diagram predicts metal oxide feasibility.
|
Card: 24 / 46 |
|
Fill in the blank: The process of combining flux with impurities during metal extraction is called ___. |
Card: 25 / 46 |
|
Basic fluxes, like CaO or MgO, are used to remove acidic impurities, while acidic fluxes, like SiO2 or B2O3, are used to remove basic impurities. |
Card: 28 / 46 |
|
Hydrogen can reduce metal oxides to metals by reacting with them at high temperatures, though its use is limited due to its flammability. |
Card: 30 / 46 |
|
The Kroll process is used for extracting titanium and zirconium from their chlorides using magnesium as a reducing agent. |
Card: 32 / 46 |
|
Fill in the blank: During electrolysis, the metal is deposited at the ___ electrode. |
Card: 33 / 46 |
|
Flux lowers the melting point of impurities, allows for easier separation from molten metal, and prevents oxidation by covering the molten metal. |
Card: 36 / 46 |
|
True or False: The reduction of sulphide ores directly to metals is thermodynamically feasible. |
Card: 37 / 46 |
|
Metal displacement method involves treating concentrated ore with a more electropositive metal to displace and extract the less electropositive metal. |
Card: 40 / 46 |
|
Describe the role of activators and depressants in the froth flotation process. |
Card: 41 / 46 |
|
Activators and depressants aid flotation.
|
Card: 42 / 46 |
|
The final step is the purification or refining of the crude metal to achieve the desired purity level. |
Card: 44 / 46 |
|
Riddle: I separate metals from their ores through chemical reactions, but without me, extraction may fail. What am I? |
Card: 45 / 46 |