|
Alcohols contain an –OH group attached to an aliphatic carbon. Phenols have an –OH group attached to an aromatic ring. Ethers consist of two alkyl or aryl groups connected by an oxygen atom (–O–). |
Card: 2 / 54 |
|
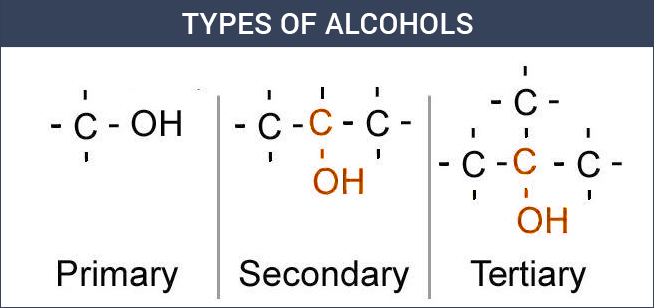
Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°) based on the carbon atom to which the –OH group is attached. |
Card: 4 / 54 |
|
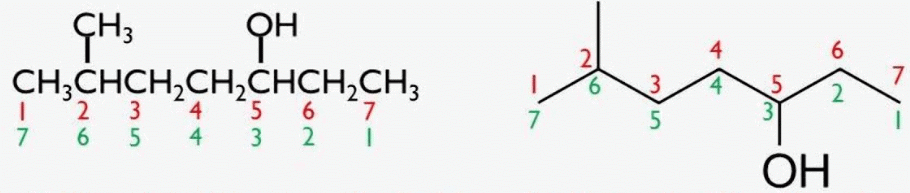
The longest carbon chain containing the –OH group is selected, and the suffix "-ol" replaces the "-e" of the parent alkane. Example: Ethanol (C2H5OH). |
Card: 8 / 54 |
|
Ethers are named as alkoxyalkanes, where the smaller group is treated as an alkoxy (–OR) substituent. Example: Methoxyethane (CH3OCH2CH3). |
Card: 12 / 54 |
|
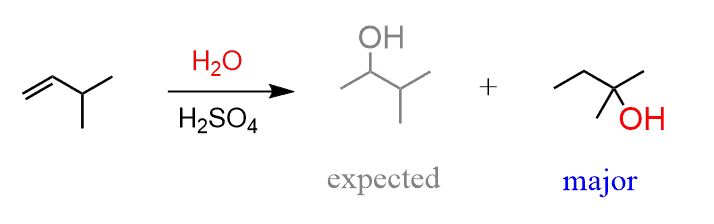
Alcohols are synthesized using: 1. Acid-catalyzed hydration (follows Markovnikov’s rule). 2. Hydroboration-oxidation (anti-Markovnikov addition). |
Card: 14 / 54 |
|
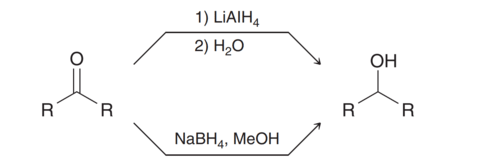
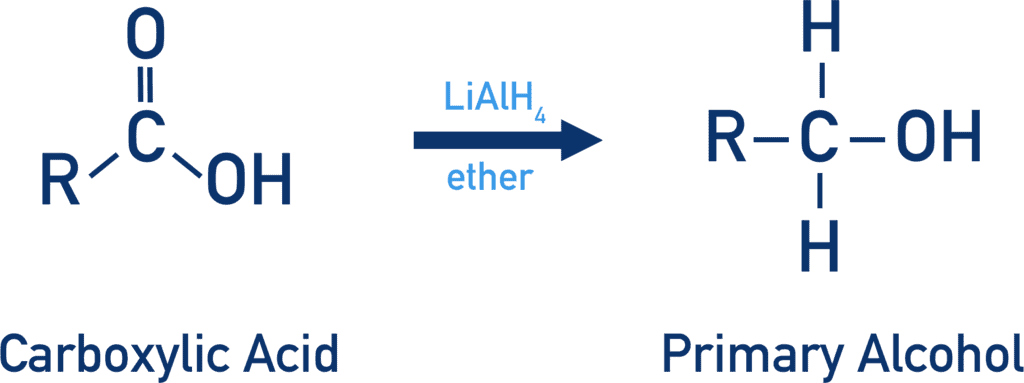
Alcohols can be obtained by reduction of aldehydes and ketones using NaBH4 or LiAlH4. |
Card: 16 / 54 |
|
Alcohols react to form alkoxides and hydrogen gas: 2C2H5OH + 2Na → 2C2H5ONa + H2 |
Card: 20 / 54 |
|
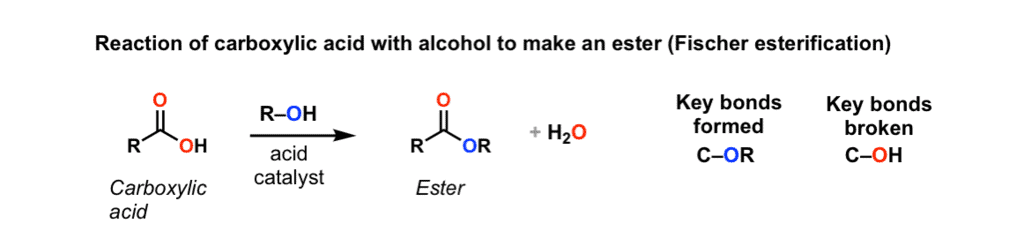
Alcohols react with carboxylic acids to form esters and water in the presence of concentrated H2SO4. |
Card: 22 / 54 |
|
Lucas test uses ZnCl2 and HCl to distinguish alcohols based on reaction time:
|
Card: 24 / 54 |
|
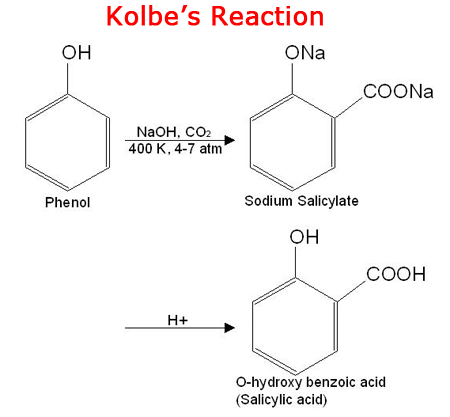
In phenols, the –OH group is attached to an aromatic ring, making it more acidic than alcohols. |
Card: 26 / 54 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Symmetrical ethers have identical alkyl/aryl groups (e.g., Diethyl ether, C2H5–O–C2H5), whereas unsymmetrical ethers have different groups (e.g., Ethyl methyl ether, C2H5–O–CH3). |
Card: 40 / 54 |
|
Ethers lack hydrogen bonding, leading to lower boiling points compared to alcohols of similar molecular weight. |
Card: 42 / 54 |
|
The –OR group activates the benzene ring, leading to ortho and para substitution in aromatic ethers like anisole. |
Card: 48 / 54 |
|
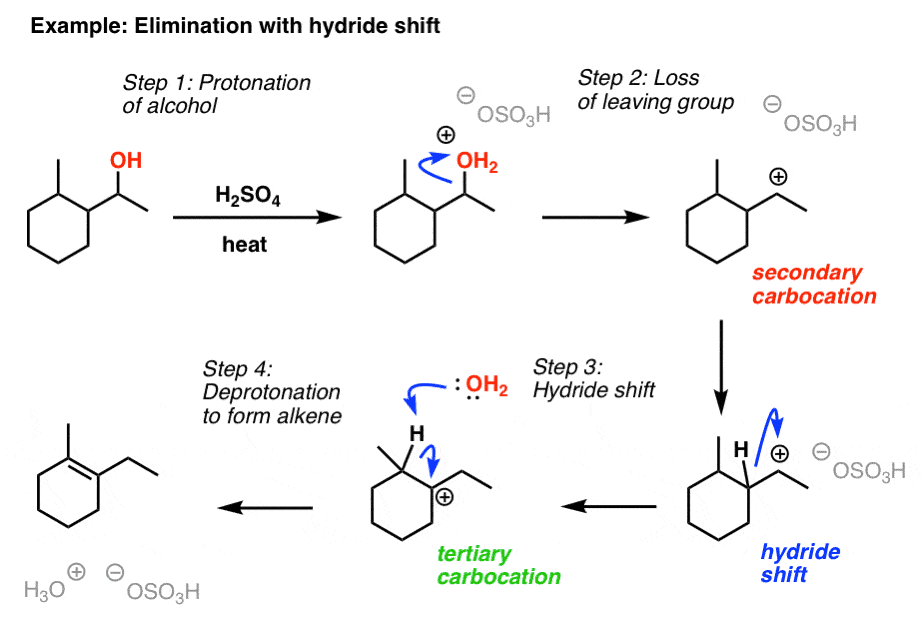
Alcohols undergo elimination (E1 mechanism) in the presence of acid (H₂SO₄), forming alkenes. |
Card: 50 / 54 |
|
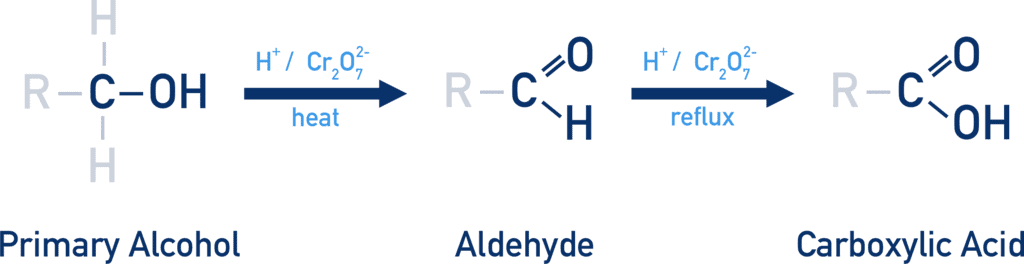
Primary alcohols → Aldehydes → Carboxylic acids (with strong oxidizers like KMnO4). |
Card: 52 / 54 |
|
Card: 54 / 54 |