The World of Metals and Non-metals Class 7 Notes Science Chapter 4 Free PDF
Introduction
Yashwant and Anandi live in a village in Rajasthan. For their school project, they decided to learn about ironsmiths—people who make useful items from metals. With their grandfather’s help, they visit Sudarshan uncle, a local ironsmith, to see how he shapes iron into everyday tools like pans, buckets, and farming equipment. Curious and amazed, they begin exploring the fascinating world of metals and how they can be shaped and used.
Curious and amazed, they begin exploring the fascinating world of metals and how they can be shaped and used.
Let’s explore the different ways metal can be used.
Properties of Materials
- Properties are characteristics that help us identify and classify materials as metals or non-metals, such as their appearance, hardness, or ability to conduct heat.
- Now, let us explore how metals and non-metals show different behaviours in these properties.
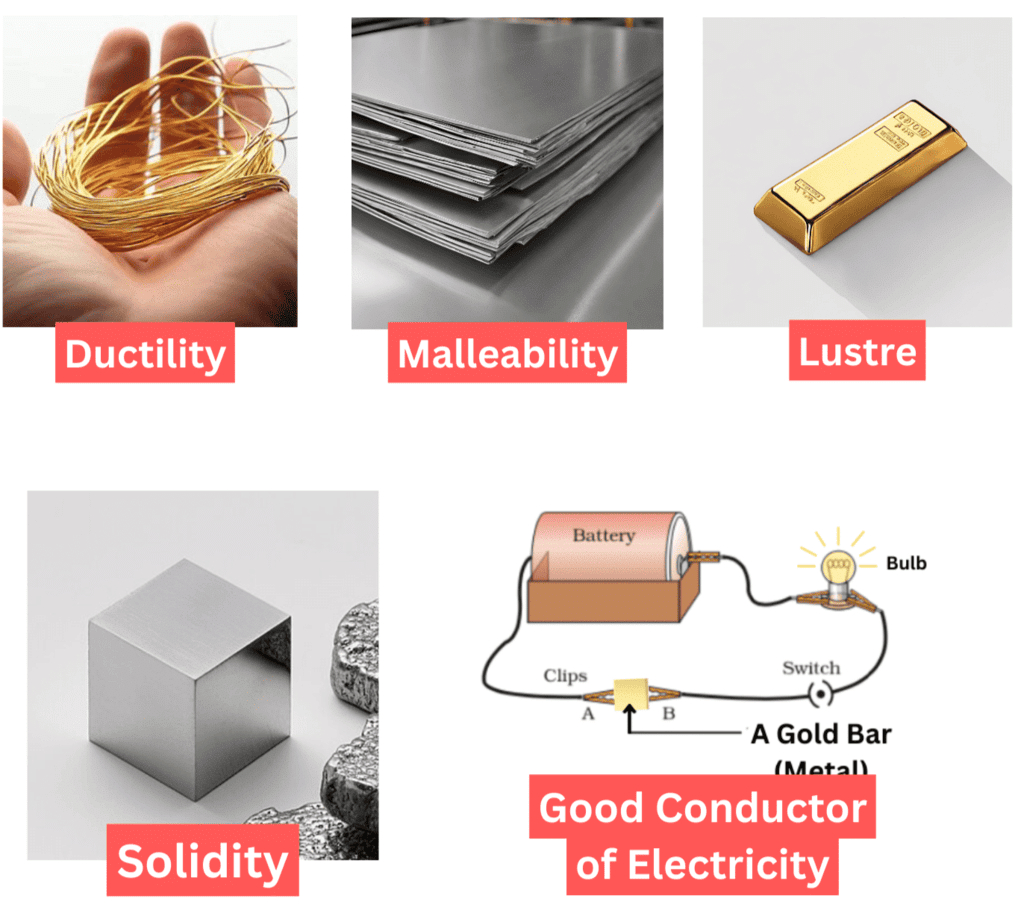
1. Malleability
Malleability is the property of materials that allows them to be beaten into thin sheets without breaking.
Beating an iron nail with a hammer
Malleability of Metals
1. Most metals, like copper, aluminium, and iron, are malleable. For example, copper and aluminium can be flattened into sheets, and iron is shaped into tools like axes.
2. Malleability is very useful in everyday life.
For example:
- Silver foil used to decorate sweets is made by hammering silver into extremely thin sheets.
- Aluminium foil, which is commonly used for wrapping food, is produced due to aluminium’s malleability.
3. Some metals, like gold and silver, are exceptionally malleable, allowing them to be made into very thin sheets called gold leaf or silver leaf.
Some Exceptions: Soft Metals and Unique States
Not all metals are hard; some metals, like sodium and potassium, are very soft and can be easily cut using a knife. These metals are much softer than copper or iron.
Another special metal is mercury, which is liquid at room temperature, unlike most metals that are solids.
Mercury’s liquid state makes it unique and useful in devices like thermometers and barometers.
Q: How does malleability benefit everyday life?
 View Answer
View Answer 
Brittleness in Non-Metals
- Unlike metals, non-metals such as coal and sulfur do not flatten when struck, which means they are not malleable.
- Instead, they tend to break or shatter into pieces.

- This characteristic is called brittleness.
- Brittle materials cannot be bent or shaped easily; they break under pressure or impact.
Properties of Wood
Wood behaves differently from both metals and brittle non-metals:
Wood does not flatten like metals when hit with a hammer.
At the same time, wood does not break easily like brittle non-metals.
Therefore, wood is considered neither malleable nor brittle, possessing some flexibility and toughness.
2. Ductility
Ductility is the property of materials that allows them to be drawn into thin wires.
Ductility of Metals
- Most metals, like copper and aluminium, are ductile.
- They are used in electrical wires and ornaments like bangles and necklaces.
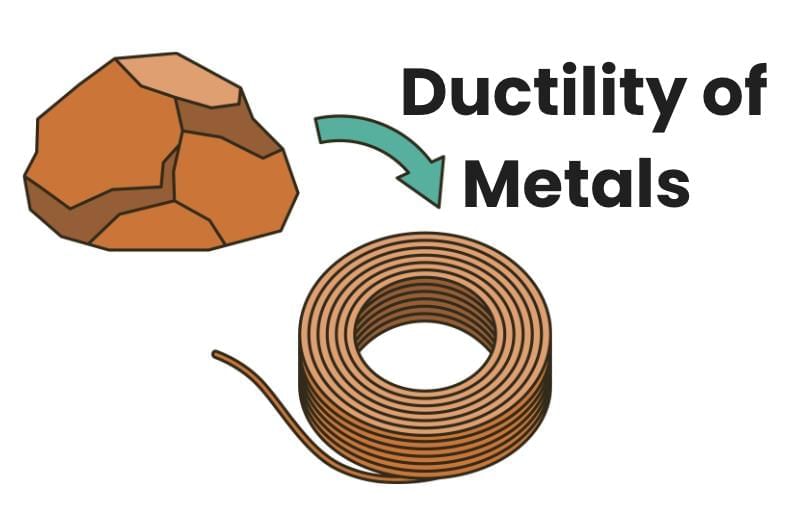
- Gold is highly ductile, with one gram drawable into a 2-kilometer-long wire.
Everyday Uses of Ductility of Metals
Electrical Fittings:
Metal wires like copper and aluminium are commonly used in electrical wiring because they conduct electricity well. You might have seen them in homes, appliances, and other electrical devices.
Jewellery and Ornaments:
Many ornaments such as bangles, necklaces, and earrings are made from metal wires. These wires are shaped and twisted to create beautiful designs.
Musical Instruments:
Metal wires are also used in many stringed musical instruments like the veena, sitar, violin, and guitar. The wires produce sound when plucked or bowed.
Ductility of Non-Metals
- Non-metals are not ductile. They break easily when stretched.
- They cannot be drawn into wires.
- Examples:
Coal, sulfur, and phosphorus break into pieces when we try to stretch or hammer them.
These non-metals are brittle, not ductile.
Dive Deeper: Steel Wires and Their Uses:
Steel is an alloy made from iron (metal) and carbon (non-metal). Steel wires are very strong and can support heavy loads. Due to their strength, steel wire ropes are used in important structures like suspension bridges. They are also used in cranes to lift heavy objects safely.
Q: What does ductility refer to in materials science?
 View Answer
View Answer 
Ductility refers to a material's ability to deform under tensile stress, often characterized by its capacity to be stretched into a wire.
3. Sonority
This ability of metals to produce ringing sounds is called sonority. Metals are described as sonorous materials because they can create loud and resonant sounds.
- Metals: Metals like those in spoons or coins produce a ringing sound, making them sonorous. This is why school bells and ghungroos (dance bells) ring.
- Non-metals: Coal and wood produce dull sounds when dropped, so they are not sonorous.
Everyday Examples of Sonority
The ringing sound of ghungroos (the small bells worn by dancers) is because of the sonorous nature of metals.

The school bell produces its loud ringing sound due to the sonority of the metal it is made from.

People can also use the difference in sound between hitting wood or metal to help navigate or identify objects, like using a stick to find their way by the sound it makes when it hits different materials.
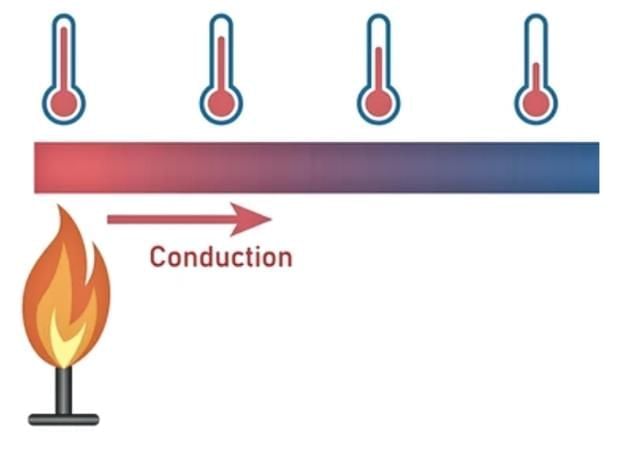
4. Conduction of Heat
Conduction is the transfer of heat through a material from one point to another.
- Metals: Metals like those used in cooking vessels (e.g., aluminium, copper, iron) are good conductors of heat, transferring heat quickly to cook food.
- Non-metals: Wood is a poor conductor of heat, staying cooler than metal when placed in hot water, which is why vessel handles are made of wood or other poor conductors.
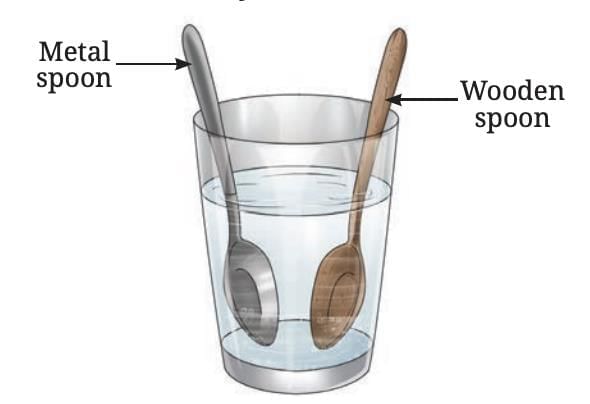
For example, if a metal spoon and a wooden spoon are both left in hot water for the same time, the metal spoon feels much hotter to the touch.
This is because the metal conducts heat faster from the hot water to your hand, while wood does not.
Metal and wooden spoons immersed in hot water
That’s why vessel handles are often made of wood or plastic—so that we can hold them safely without getting burned.
Good and Poor Conductors
The process of heat moving through a material from one point to another is called conduction.
Materials that allow heat to pass through them easily are known as conductors. Metals fall in this category.
On the other hand, materials like wood do not transfer heat well and are known as poor conductors or insulators.
5. Conduction of Electricity
Materials that allow electricity to flow easily are good conductors of electricity, while those that don’t are poor conductors.
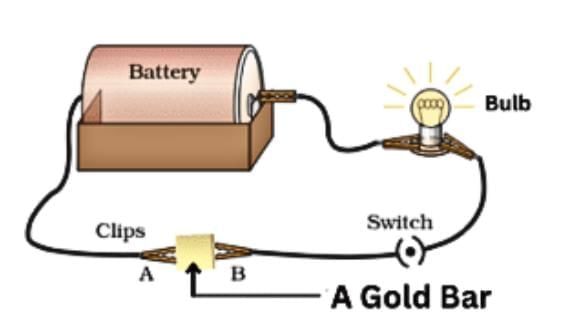
- Metals: Aluminium, iron, and copper are good conductors, making bulbs glow in a tester circuit, used for electrical wires.
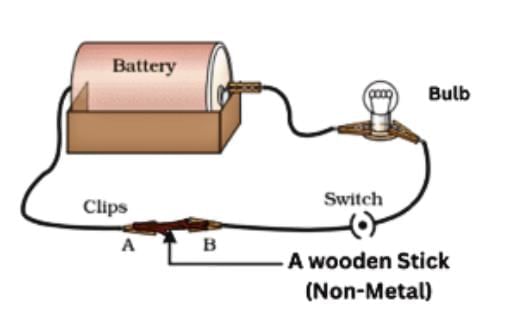
- Non-metals: Sulfur, coal, wood, stone, rubber, and nylon are poor conductors, not allowing bulbs to glow, used for insulating materials like screwdriver handles and electrician’s gloves.
Electrical Conductivity and Safety
For example, the handle of a screwdriver used by electricians is often made of plastic or rubber, which are poor conductors of electricity.
Electricians also wear rubber gloves and rubber-soled shoes while working. This is because rubber does not allow electricity to pass through easily, protecting them from electric shocks.
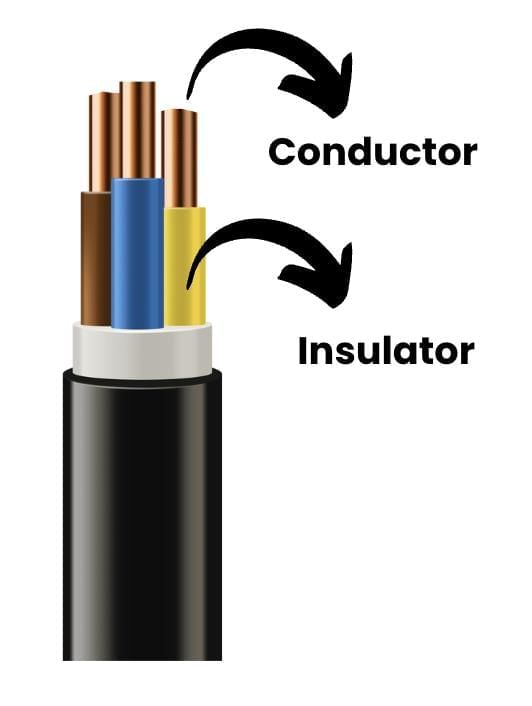
Conductors and Insulators
Materials that allow electricity to flow through them easily are called good conductors of electricity.
Metals such as aluminium, iron, and copper are excellent conductors and are widely used in electrical wiring and devices.
- On the other hand, materials like sulfur, coal, wood, stone, rubber, and nylon do not allow electricity to flow freely and are called poor conductors of electricity or insulators.
6. Metallic Lusture
Metals such as copper, aluminium, and iron have a characteristic shiny appearance known as metallic lustre. This means they reflect light well, giving them a bright, polished look.

These metals are generally hard, meaning they resist scratching or denting under normal conditions.
In contrast, non-metals like coal, sulfur, and wood do not have this shiny appearance. Instead, they look dull or matte because they do not reflect light like metals.

Non-metals are usually softer compared to metals, meaning they can be scratched or broken more easily.
HOLISTIC LENS: The impact of iron on the progress of the civilisation of India
In ancient India, early civilizations like the Harappans used metals such as copper and gold to make tools and jewellery. However, the widespread use of iron came much later. Iron became important because of its strength and durability. Iron tools, especially agricultural implements like ploughs, greatly helped improve farming and contributed to the progress of Indian civilization.
Why do you think copper was discovered and used before iron?Copper was discovered before iron because it occurs naturally in a pure form and has a lower melting point, making it easier to find and work with using early technology. Iron, found mostly as ore, requires higher temperatures and more advanced tools to extract and shape.
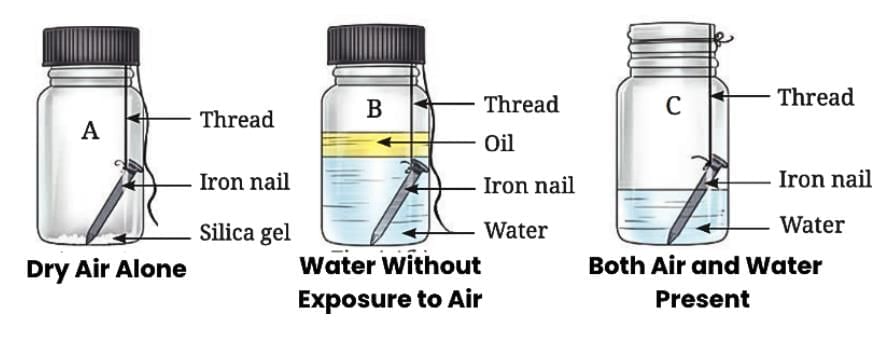
Effect of Air and Water on Metals: Iron
Rusting of Iron:
When iron objects are left exposed to the environment, they often develop brown deposits on their surface. This phenomenon is called rusting.
Rusting Iron
The brown deposits that form on iron are called rust.
Rusting is a chemical reaction where iron reacts with oxygen and moisture in the air.
This reaction causes the iron to deteriorate and weaken over time.
Conditions for Rusting:
Rusting happens only when iron comes into contact with both air and water (moist air).
Iron does not rust if it is exposed to dry air alone.
Iron also does not rust if it is submerged in water without exposure to air.
- Therefore the process of rust formation, called rusting, requires both air and water, making moist air the cause of the brown deposits.
Impact
Rusting causes iron objects and structures to become weak and unsafe.
It leads to damage and decay of iron used in buildings, vehicles, bridges, and tools.
In many countries, including ours, a large amount of money is spent every year on repairing or replacing rusted iron.
Prevention of Iron from Rusting
Rusting can be prevented by several methods, such as:
Painting iron surfaces to keep air and moisture away.
Applying oil or grease to form a protective layer.
Galvanisation: Coating iron with a layer of zinc to protect it.
Q: What role do air and water play in the corrosion of iron?
 View Answer
View Answer 
Air and water contribute to the oxidation process that leads to the corrosion of iron.
Corrosion
Corrosion is the gradual deterioration of metal surfaces due to air, water, or other substances.
Fascinating Fact: The Iron Pillar of Delhi
The Iron Pillar of Delhi was built over 1600 years ago during Chandragupta II’s time. It is 8 metres tall and weighs more than 6000 kilograms.
Despite facing rain, wind, and weather for centuries, it has hardly rusted. This shows how skilled ancient Indian metalworkers were in making strong and lasting iron.
Effect of Air and Water on Other Metals
1. Magnesium
- Reaction with Air: When a magnesium ribbon is burned, it produces a dazzling white flame and turns into a white powder called magnesium oxide.

- Nature of Oxide: Mixing magnesium oxide with warm water and testing with litmus shows it turns red litmus blue, indicating it is basic in nature.
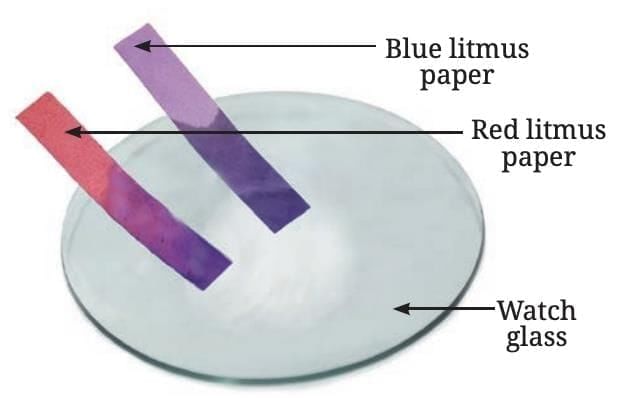
- General Rule: Oxides of metals are generally basic.
2. Sodium
- Storage: Sodium is stored in kerosene to prevent it from reacting with oxygen and water in the air, as it reacts vigorously, producing a lot of heat.
- Oxide Nature: Sodium oxide is basic, like other metal oxides.
Substances that Behave Differently from Metals in Air and Water
Certain substances, such as sulfur and phosphorus, behave very differently from metals when exposed to air and water.
Sulfur
- Reaction with Air: When sulfur is burned, it produces sulfur dioxide gas, which, when dissolved in water, forms sulfurous acid.
- Nature of Oxide: Testing the solution with litmus shows it is acidic, turning blue litmus red.
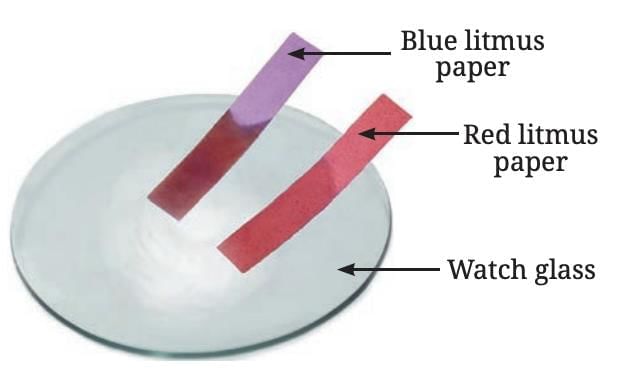
- Reaction with Water: Sulfur does not react with water when mixed, unlike some metals.
Phosphorus
- Storage: Phosphorus is stored in water because it catches fire when exposed to air.
Properties of Non-metals:
- Usually soft and dull (non-lustrous) in appearance.
- Not malleable or ductile, so they cannot be shaped into sheets or wires.
- Not sonorous, producing dull sounds when struck.
- Poor conductors of heat and electricity.
- Oxides are acidic, unlike the basic oxides of metals.
- Examples: Oxygen, hydrogen, nitrogen, carbon, chlorine, iodine.
- Materials like plastic, glass, wood, rubber, and paper are not classified as metals or non-metals because they are not elements.
Dive Deeper: Elements (The Building Blocks of Matter)
Elements are pure substances that cannot be broken down into simpler substances by ordinary chemical methods.
Everything around us is made up of these elements.
There are currently 118 known elements.
Some elements occur naturally in the environment, such as oxygen, iron, and gold.
Others are artificially created in laboratories and do not exist naturally.
Metals and non-metals are two important sub-categories of elements.
You will learn more about elements and their properties in higher classes.
Are Non-Metals Essential in Everyday Life?
While metals are very visible in daily life due to their shiny appearance, strength, and ability to conduct heat and electricity, non-metals play equally vital roles in our lives.
Importance of Non-metals
1. Oxygen is a non-metal that we breathe every day. Without oxygen, life on Earth would not survive.
Other uses of oxygen include:
Used in hospitals to assist patients who have difficulty breathing.
Used in welding and combustion processes.
2. Carbon is essential because it is the building block of all life forms.
It is a key part of proteins, fats, and carbohydrates which provide energy and help in growth.
3. Nitrogen is a non-metal widely used in the manufacture of fertilizers and chemicals.
It is a vital nutrient that helps plants grow healthy and strong.
4. Chlorine is commonly used in water purification to make drinking water safe.
5. Iodine solution is applied on wounds as an antiseptic to prevent infections.
Science and Society
1. Many metals and alloys (mixtures of two or more metals or metals with non-metals) are used daily in utensils, tools, and machines.
2. Metals are essential in modern technology and almost every industry, including special fields like:
Atomic energy (e.g., zirconium)
Aerospace (e.g., titanium)
3. In India, recycling metals like iron and aluminium is common and helps to reduce waste and promote sustainability.
Points to Remember
Metals are shiny, hard, bendable (malleable), stretchable (ductile), make ringing sounds (sonorous), and conduct heat and electricity well.
Non-metals are usually dull, soft or brittle, and do not conduct heat or electricity well.
Malleability lets metals like copper and gold be made into thin sheets (foils, tools).
Ductility lets metals be drawn into wires for electrical use and jewelry.
Metals produce clear ringing sounds used in bells, while non-metals produce dull sounds.
Metals conduct heat, so they are used for cooking pots; non-metals like wood are used for handles because they don’t conduct heat.
Metals like copper and aluminium conduct electricity, while non-metals like rubber and plastic act as insulators for safety.
Iron rusts when exposed to both air and water, forming reddish-brown rust.
Copper and silver also get coated when exposed to air and moisture (green and black coatings).
The Iron Pillar of Delhi (1600+ years old) shows how ancient Indians made iron that doesn’t rust easily.
Magnesium burns in air forming a basic oxide; sodium is stored in kerosene to prevent reaction with air.
Sulfur burns to form sulfur dioxide, which forms acidic sulfurous acid when dissolved in water, but sulfur alone doesn’t react with water.
Non-metals like phosphorus are stored in water to stop them catching fire in air; their oxides are acidic.
Non-metals are important: oxygen for breathing, carbon for life, nitrogen for fertilizers, chlorine to clean water, iodine as medicine.
There are 118 elements, divided into metals and non-metals, some natural and some made in labs.
Metals and alloys are used in tools, machines, and industries; recycling metals like iron and aluminium helps protect the environment.
Q: What properties of metals allow them to be used in making electrical wires and tools?
 View Answer
View Answer 
Malleability and ductility are the properties that allow metals to be shaped into tools and drawn into wires.
Difficult Words and Their Meanings
- Malleability: The ability of a material, like metal, to be beaten into thin sheets without breaking.
- Ductility: The ability of a material, like metal, to be drawn into thin wires.
- Sonority: The property of a material, like metal, to produce a ringing sound when struck.
- Conduction: The transfer of heat or electricity through a material, like heat through a metal spoon.
 Metal Characteristics
Metal Characteristics - Conductor: A material, like copper, that allows heat or electricity to flow easily.
- Insulator: A material, like rubber, that does not allow heat or electricity to flow.
- Rusting: The process where iron forms brown deposits (rust) when exposed to both air and water.
- Corrosion: The gradual damage to a metal’s surface by air, water, or other substances, like rusting or green coating on copper.
- Oxide: A substance formed when a metal or non-metal reacts with oxygen, like magnesium oxide or sulfur dioxide.
- Element: A basic substance, like iron or oxygen, that cannot be broken into simpler substances, with 118 known types.
|
80 videos|319 docs|12 tests
|
FAQs on The World of Metals and Non-metals Class 7 Notes Science Chapter 4 Free PDF
| 1. What are the main differences between metals and non-metals? |  |
| 2. How do metals and non-metals react with oxygen? |  |
| 3. What are some common uses of metals and non-metals? |  |
| 4. How can we identify metals and non-metals in everyday life? |  |
| 5. What safety precautions should be taken while handling metals and non-metals? |  |