Year 9 Exam > Year 9 Notes > Year 9 Science IGCSE (Cambridge) > Chapter Notes: Reactivity
Reactivity Chapter Notes | Year 9 Science IGCSE (Cambridge) PDF Download
Reactivity and Displacement Reactions
 Chemical Vibrance
Chemical Vibrance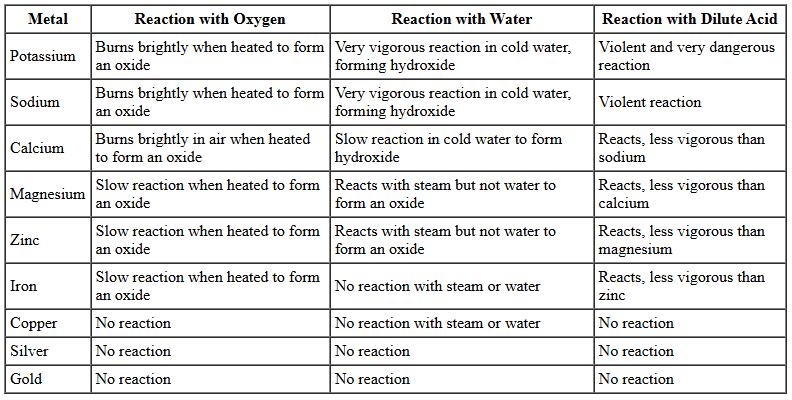
- The reactivity series and displacement reactions are important concepts in understanding how metals interact with other substances based on their chemical reactivity. This has implications in both industrial and laboratory settings.
The Reactivity Series
- The reactivity series ranks metals according to their reactivity, with the most reactive metals at the top and the least reactive at the bottom: Potassium (K), Sodium (Na), Calcium (Ca), Magnesium (Mg), Zinc (Zn), Iron (Fe), Copper (Cu), Silver (Ag), Gold (Au).
- Reactivity is determined by how metals react with oxygen, water (or steam), and dilute acids.
- As you move down the series, reactivity decreases. For example, potassium reacts very vigorously, while gold is unreactive.
Displacement Reactions
- A displacement reaction occurs when a more reactive metal replaces a less reactive metal in a compound, such as a salt.
- For example, when an iron nail is placed in a copper sulfate solution, the blue colour of the solution fades.
- This happens because iron, being more reactive than copper, displaces copper from the copper sulfate solution.
- The reaction produces iron sulfate and coats the nail with copper.
- The word equation for this reaction is: copper sulfate plus iron yields iron sulfate plus copper.
- The symbol equation is: CuSO₄ + Fe → FeSO₄ + Cu.
- If a copper nail is placed in iron sulfate, no reaction occurs because copper is less reactive than iron and cannot displace it.
Using the Reactivity Series and Displacement Reactions
Displacement Reactions
 Iron Production
Iron Production - Displacement reactions are a way to identify unknown metals by seeing if they can replace known metals in their salts.
- In industrial processes, aluminium, which is more reactive than iron, can displace iron from iron oxide. This reaction produces aluminium oxide and iron.
- This process is called the thermite reaction, which is exothermic, meaning it releases a large amount of energy and produces molten iron, which has a melting point of 1535 °C.
- The thermite reaction is used for welding railway rails on-site, where molten iron is used to join the rails together.
- This reaction is initiated by an exothermic reaction between magnesium powder and barium nitrate.
- Although carbon is not a metal, it can displace metals like zinc, iron, tin, and lead from their ores, which are metal compounds found in rocks.
- Historically, around 3500 years ago, carbon in the form of charcoal was used to displace iron from iron ore, which is iron oxide.
- In modern times, iron ore (iron oxide) and coke (carbon) are heated with air in a blast furnace to produce molten iron and carbon dioxide. This reaction can be represented as:
- iron oxide + carbon → iron + carbon dioxide.
Salts
- Salts are chemical compounds formed through reactions involving acids, and they have various applications in daily life and industry.
What is a Salt?
- Salts encompass compounds like sodium chloride (table salt), copper sulfate, silver nitrate, and calcium carbonate, each serving distinct purposes.
- Sodium chloride is used for food preservation and flavour enhancement.
- Magnesium carbonate aids gymnasts by keeping their hands dry to prevent slipping.
- Calcium sulfate is utilized in the production of blackboard chalk.
- Aluminium sulfate assists in the adherence of dyes to fibres.
- Copper sulfate is employed to coat soya seeds, preventing fungal growth during planting.
- Ammonium nitrate serves as a fertilizer to promote crop growth.
Acids and Salts
Introduction
 Chemical Reactions
Chemical Reactions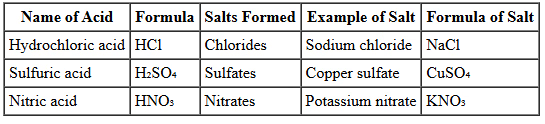
- The chemical industry produces a large amount of salts, often starting with acids that contain hydrogen. In the laboratory, common acids and the salts they produce include:
- Carbonic acid, which is weak and formed from carbon dioxide and water, produces carbonates.
- Citric acid, found in fruits like oranges and lemons, produces citrates.
Salts are compounds that result from the reaction between an acid and a base. In the chemical industry and laboratories, salts are produced in large quantities through various reactions, often starting with acids. Here are some common acids and the salts they produce:
- Carbonic Acid: This is a weak acid formed from the reaction of carbon dioxide with water. Carbonic acid produces carbonates, which are salts containing the carbonate ion (CO₃²⁻). Carbonates are commonly found in nature and are used in various industrial applications.
- Citric Acid: Citric acid is a natural acid present in citrus fruits like oranges and lemons. It is used to produce citrates, which are salts containing the citrate ion. Citrates have various uses in food preservation, pharmaceuticals, and as dietary supplements.
Making Salts Using a Metal and an Acid
- Reacting metals with dilute acids is a common method for producing salts.
- The general equation is: acid + metal → salt + hydrogen.
- Example: Zinc reacts with hydrochloric acid to form zinc chloride and hydrogen.
- Word equation: zinc + hydrochloric acid → zinc chloride + hydrogen.
- Symbol equation: Zn + 2HCl → ZnCl₂ + H₂.
Making Salts Using a Metal Oxide
- Unreactive metals like silver and copper do not react with acids to form salts, so metal oxides are used instead.
- General equation: metal oxide + acid → salt + water.
- Example: Heating copper oxide with sulfuric acid produces copper sulfate and water.
- Word equation: copper oxide + sulfuric acid → copper sulfate + water.
- Symbol equation: CuO + H₂SO₄ → CuSO₄ + H₂O.
Other Ways of Making Salts
- Salts can be produced through various chemical reactions, including those with metal carbonates, neutralisation with alkalis, and others.
- Each method yields specific salts with practical applications.
Carbonates
 Coral Erosion
Coral Erosion - Carbonates are formed when a metal reacts with carbonic acid, resulting in salts like calcium carbonate.
- When carbonates react with acids, they produce new salts, along with water and carbon dioxide.
- For example, when sulfuric acid reacts with calcium carbonate, it produces calcium sulfate, water, and carbon dioxide.
- Word equation: sulfuric acid + calcium carbonate → calcium sulfate + water + carbon dioxide.
- Symbol equation: H₂SO₄ + CaCO₃ → CaSO₄ + H₂O + CO₂.
- Similarly, hydrochloric acid reacts with calcium carbonate to form calcium chloride, water, and carbon dioxide.
- Word equation: hydrochloric acid + calcium carbonate → calcium chloride + water + carbon dioxide.
- Symbol equation: 2HCl + CaCO₃ → CaCl₂ + H₂O + CO₂.
- The general equation for acid-carbonate reactions is: acid + carbonate → salt + water + carbon dioxide.
- Limestone, which is primarily calcium carbonate, undergoes erosion when it reacts with acid rain.
- Coral skeletons, composed of calcium carbonate, are affected by more acidic ocean waters, which result from increased carbon dioxide levels. This reaction leads to the degradation of coral.
Salts in Rocks
- Rocks can contain identifiable salts, which are often indicated by their colours. For example, blue-green rocks found in the Atacama Desert in Chile are known to contain copper salts.
- One such copper salt is malachite, a bright blue-green mineral that is a form of copper carbonate and is commonly found in these rocks.
Forming Salts by Neutralisation
- Neutralisation is the chemical process in which an acid reacts with a base to produce a salt and water as the main products.
Neutralization
 Vibrant Chemistry
Vibrant Chemistry - Neutralization happens when an alkali reacts with an acid, producing a salt and water. For example, when sodium hydroxide reacts with hydrochloric acid, it forms sodium chloride and water.
- Word equation: sodium hydroxide + hydrochloric acid → sodium chloride + water.
- Symbol equation: NaOH + HCl → NaCl + H₂O.
- The general equation for neutralization is: acid + alkali → salt + water.
Alkalis and Bases
- Metal oxides are bases. When they dissolve in water, they create alkaline solutions called alkalis. For instance, sodium oxide dissolves in water to form sodium hydroxide, which is an alkali.
- Word equation: sodium oxide + water → sodium hydroxide.
- Insoluble metal oxides, such as iron oxide and copper oxide, do not produce alkalis when dissolved in water. However, they can react with acids to form soluble salts. For example, copper oxide reacts with sulfuric acid to produce copper sulfate and water.
- Word equation: copper oxide + sulfuric acid → copper sulfate + water.
- Symbol equation: CuO + H₂SO₄ → CuSO₄ + H₂O.
Rearranging Atoms
- During chemical reactions, atoms are rearranged to form new combinations, but no atoms are lost or created. For instance, when iron reacts with sulfur, they form iron sulfide by rearranging the iron and sulfur atoms.
- Word equation: iron + sulfur → iron sulfide.
- Symbol equation: Fe + S → FeS.
- In all reactions, the elements present in the reactants are also found in the products, just in different combinations. For example, when magnesium reacts with hydrochloric acid, it produces magnesium chloride and hydrogen.
- Symbol equation: Mg + 2HCl → MgCl₂ + H₂.
- Analysis: One magnesium atom is in the reactants and products (in MgCl₂); two hydrogen atoms are in the reactants (in 2HCl) and products (as H₂); two chlorine atoms are in the reactants (in 2HCl) and products (in MgCl₂).
Conservation of Mass
- The conservation of mass principle states that mass is neither created nor destroyed during a chemical reaction.
Energy and Chemical Reactions
 Magnesium Combustion
Magnesium Combustion - Atoms have mass, and because no atoms are created or destroyed in a chemical reaction, the total mass remains constant.
- The law of conservation of mass states that the mass of reactants is equal to the mass of products in a closed system.
- For example, when calcium carbonate reacts with hydrochloric acid in a sealed flask, the mass stays the same because no atoms can enter or leave the system.
- When the flask is open, carbon dioxide gas escapes, making it seem like the mass decreases (for instance, from 250 g to 207 g after 10 minutes), but the total mass of all products, including the gas, is still equal to the mass of the reactants.
- In the reaction, oxygen is found in calcium carbonate (the reactant) and in water and carbon dioxide (the products). Hydrogen from hydrochloric acid (the reactant) forms water in the products.
- For instance, when magnesium is heated in a crucible with air, its mass increases because magnesium reacts with oxygen from the air to form magnesium oxide.
- In this case, the word equation is magnesium + oxygen → magnesium oxide.
- The increase in mass occurs because oxygen atoms are added to the magnesium, which may seem surprising since magnesium oxide looks lighter.
- Antoine Lavoisier’s experiments in the late 1770s demonstrated that burning involves the combination of a gas (oxygen) from the air, similar to the process of respiration, explaining the increase in mass.
Word Equations
- Calcium Carbonate + Hydrochloric Acid → Calcium Chloride + Water + Carbon Dioxide
- Magnesium + Oxygen → Magnesium Oxide
Understanding Energy Changes in Chemical Reactions
 Energy Dynamics
Energy Dynamics Chemical reactions are all about energy changes. When reactants are involved, energy is needed to break the bonds between them. Once new bonds are formed in the products, energy is released. This process follows the law of conservation of energy, which states that energy cannot be created or destroyed, only transformed from one form to another.
- Energy in Chemical Reactions:
- Breaking Bonds: When a chemical reaction occurs, energy is required to break the bonds holding the atoms together in the reactants. This is the initial step of the reaction.
- Forming Bonds: After the bonds in the reactants are broken, atoms rearrange themselves and form new bonds, creating the products of the reaction. This process releases energy.
- Energy Types:
- Thermal Energy: Heat energy that can be felt as warmth.
- Light: Energy that is visible as light.
- Sound: Energy that is heard as sound.
- Kinetic Energy: Energy of movement.
- Example - Potassium and Water:
- When potassium reacts with water, it is a very energetic reaction. Potassium is a metal that reacts vigorously with water.
- Reaction Details:
- Word Equation: Potassium + Water → Potassium Hydroxide + Hydrogen
- Symbol Equation: 2K + 2H₂O → 2KOH + H₂
- Energy Release: During this reaction, energy is released in the form of thermal energy (heat), light, and sound. The reaction is so vigorous that it can produce a pinkish-purple flame due to the intense heat and energy released.
Exothermic Reactions
- Definition: Exothermic reactions are those in which less energy is needed to break the bonds of the reactants than the energy released when the bonds of the products are formed. In simpler terms, these reactions release energy into their surroundings.
- Example - Potassium and Water: The reaction between potassium and water is a prime example of an exothermic reaction. In this reaction, a large amount of energy is released, making it highly energetic and vigorous.
Endothermic Reactions
- Definition: Endothermic reactions are those in which more energy is required to break the bonds of the reactants than the energy released when the bonds of the products are formed. These reactions absorb energy from their surroundings, usually in the form of heat.
- Example - Sodium Bicarbonate and Citric Acid: A common example of an endothermic reaction is the reaction between sodium bicarbonate (baking soda) and citric acid. This reaction is often found in products like sherbet sweets.
- Reaction Details:
- Word Equation: Sodium Bicarbonate + Citric Acid → Sodium Citrate + Water + Carbon Dioxide
- Cooling Sensation: When sodium bicarbonate reacts with citric acid, it absorbs energy from the environment, which is why you feel a cooling sensation when you consume products containing this reaction. The energy is transferred from the environment (such as your mouth) to the chemical energy stored in the bonds of the products, creating this cool feeling.
The Law of Conservation of Energy
- Principle: The law of conservation of energy states that energy cannot be created or destroyed; it can only change forms. The total amount of energy before and after a reaction remains the same.
- Energy Transformation: During a chemical reaction, energy can transform from one form to another, such as from chemical energy to thermal energy (heat). However, the total amount of energy in the system remains conserved.
- Application: This principle applies to all chemical reactions, whether they are exothermic (releasing energy) or endothermic (absorbing energy). The energy balance is maintained throughout the process.
The document Reactivity Chapter Notes | Year 9 Science IGCSE (Cambridge) is a part of the Year 9 Course Year 9 Science IGCSE (Cambridge).
All you need of Year 9 at this link: Year 9
|
2 videos|39 docs|9 tests
|
FAQs on Reactivity Chapter Notes - Year 9 Science IGCSE (Cambridge)
| 1. What is a reactivity series and how does it relate to displacement reactions? |  |
Ans. A reactivity series is a list of metals arranged in order of their decreasing reactivity. It helps predict how metals will react in displacement reactions. In a displacement reaction, a more reactive metal can displace a less reactive metal from its compound. For example, if zinc is placed in a solution of copper sulfate, zinc will displace copper because it is higher in the reactivity series.
| 2. How can salts be formed through neutralization reactions? |  |
Ans. Salts can be formed through neutralization reactions, which occur when an acid reacts with a base. In this process, the hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base to form water (H2O), and the remaining ions form a salt. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), sodium chloride (NaCl) and water are produced.
| 3. What are some common methods for making salts other than neutralization? |  |
Ans. Other than neutralization, salts can also be made through various methods such as direct combination, where elements react directly to form a salt (e.g., sodium and chlorine forming sodium chloride), and precipitation reactions, where two soluble salts react in solution to form an insoluble salt that precipitates out (e.g., mixing silver nitrate and sodium chloride to form silver chloride).
| 4. What role do energy changes play in chemical reactions? |  |
Ans. Energy changes are crucial in chemical reactions as they determine whether a reaction is exothermic or endothermic. In exothermic reactions, energy is released, often in the form of heat, while in endothermic reactions, energy is absorbed. Understanding these energy changes helps predict the feasibility and spontaneity of reactions.
| 5. Why is it important to understand the concept of reactivity in practical applications? |  |
Ans. Understanding the concept of reactivity is important for practical applications such as metallurgy, where it helps in selecting the right metals for specific processes, and in predicting the outcomes of chemical reactions in industries like pharmaceuticals and agriculture. It also aids in safety protocols, ensuring that more reactive substances are handled appropriately to prevent unwanted reactions.
Related Searches
















