Nature of Matter: Elements, Compounds, and Mixtures Chapter Notes | Science Curiosity Class 8 - New NCERT PDF Download
Introduction
What is everything around you really made of?
- From the air you breathe to your food, your clothes, and even your school books—almost everything is matter made of tiny particles!
- But most things aren’t made from just one pure substance; instead, they’re created by combining different kinds of particles in various ways.
- In this chapter, you'll explore how the world is built from elements, compounds, and mixtures, and discover how new combinations can even help solve big problems like cleaning our air.
What Are Mixtures?
A mixture is formed when two or more substances are combined such that each substance keeps its own properties and does not react chemically with the others. The individual substances in a mixture are called its components.
Types of Mixtures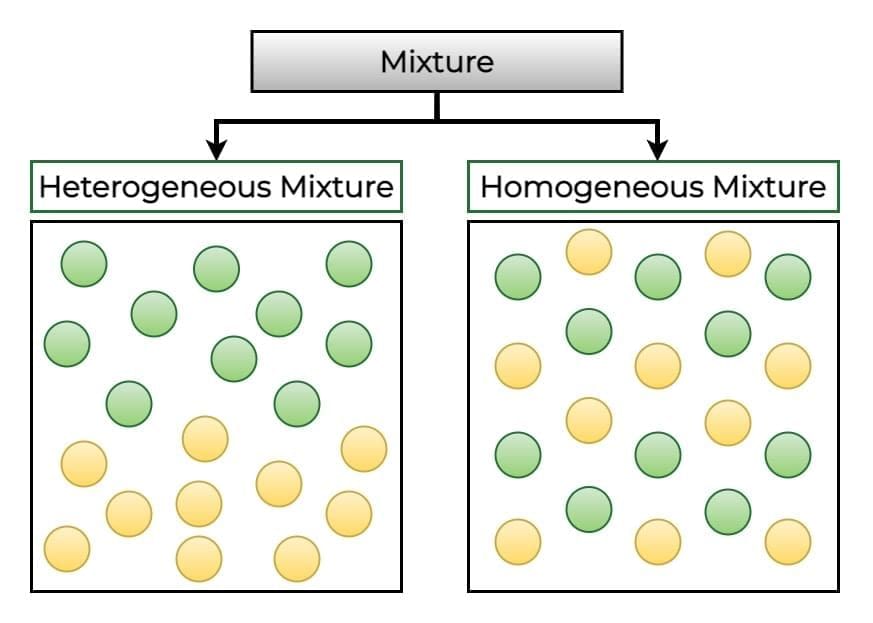
Non-uniform (Heterogeneous) Mixtures:
You can see and often separate each component easily.
Examples: Poha, sprout salad, mixture of sand and salt, trail mix, and the components in vegetable soup.
Uniform (Homogeneous) Mixtures:
Components are so well mixed you cannot see them separately—even under a microscope.
Examples: Sugar dissolved in water, saltwater, air, or alloys like stainless steel.
- Mixtures are common in daily life: Most things we use—food, air, beverages—are actually mixtures.
- Properties: The properties of a mixture depend on the proportion of its components, which can vary.
Scientific Heritage: Alloys
- Alloy (Mishraloha): An ancient Indian mixture of two or more metals with distinct properties.
- Examples: Bronze (copper + tin), Brass (copper + zinc), and Stainless Steel (iron + nickel + chromium + carbon).
- Alloys are typically uniform (homogeneous) mixtures.
Is Air a Mixture?
Air is a uniform mixture of gases: mainly nitrogen, oxygen, argon, carbon dioxide, and water vapour.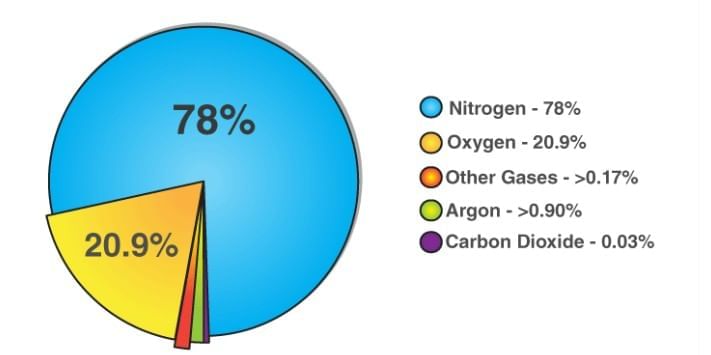 Components of AirWhy is air a mixture?
Components of AirWhy is air a mixture?- The gases in air are not chemically bonded; each gas keeps its own properties.
- Air is uniform throughout (homogeneous mixture).
- You cannot see or separate the gases by sight.
Testing for Carbon Dioxide in Air
Add calcium oxide (quick lime) to water to get lime water (solution of calcium hydroxide).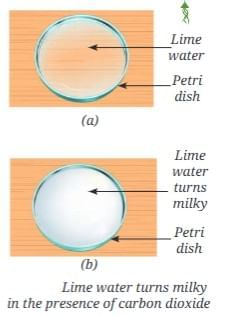 Expose lime water to air:
Expose lime water to air:
- After some time, the clear solution turns milky.
- This happens because carbon dioxide in the air reacts with calcium hydroxide to produce insoluble calcium carbonate (which looks milky)
- Conclusion: The air around us contains carbon dioxide.
Mixtures Can Have Other Components (Dust, Pollutants): Dust Particles in Air
- Leave a clean black sheet of paper by an open window or outside for a few hours.
- Observe dust particles settling on its surface (examine with a magnifying glass for detail).
- Conclusion: Dust and other particulates are present in air, though their amount varies with location and time. These are considered pollutants.
- Major air pollutants include particulate matter (dust, soot) and gases (carbon monoxide, ozone, NO₂, SO₂). Air quality is described using the Air Quality Index (AQI).
Types of Mixtures
Mixtures can be made by mixing substances in any physical state (solid, liquid, gas).Table of Mixture Types (with examples):
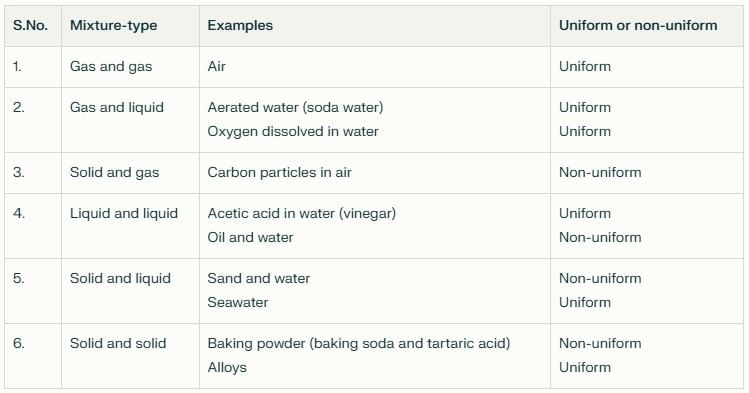
- In daily life: To get a component you want (e.g., seeds from fruit, stones from rice).
- In science: To obtain pure substances for further study or use.
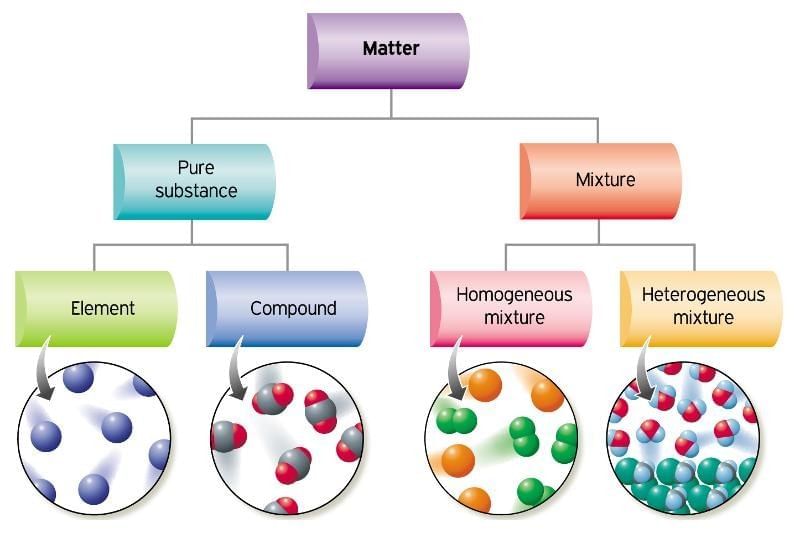
What Are Pure Substances?
Everyday Meaning:
- When you see “pure” on a packet of milk, ghee, or spices, it usually means the product is unadulterated, i.e., it does not have harmful or cheaper substances mixed in.
- Adulteration is the illegal act of mixing lower-quality or harmful substances into foods or products to increase quantity or cut costs, but this reduces quality and can be dangerous to health.
Scientific Definition of a Pure Substance
- Contains only one kind of matter (one type of particle) throughout.
- Examples: sugar, distilled water, baking soda (chemically pure), pure iron, pure gold (24K).
- A pure substance cannot be separated into other substances by any physical process (like filtering, sieving, or evaporation).
- If something is made of more than one kind of substance (like milk, soil, packed juice), science classifies it as a mixture, not a pure substance.
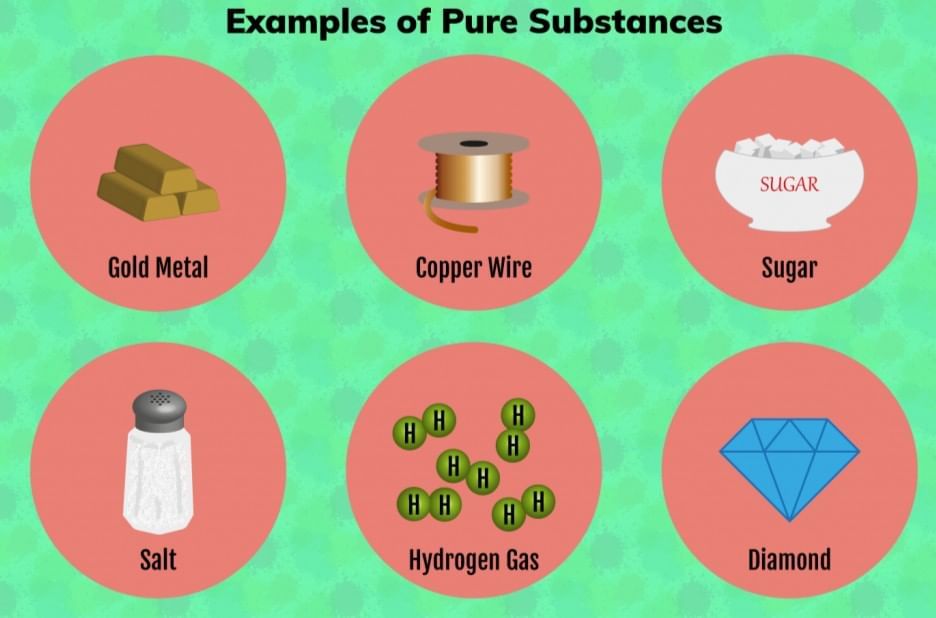
Q. Classify the following as mixture or pure substance: Milk, Packaged fruit juice, Baking soda, Sugar, Soil.
- Milk: Mixture (contains water, fats, proteins, etc.)
- Packaged fruit juice: Mixture (water, sugars, flavors, vitamins, etc.)
- Baking soda: Pure substance (if chemically pure—only sodium bicarbonate)
- Sugar: Pure substance (if only sucrose)
- Soil: Mixture (sand, clay, minerals, organic matter, water, air)
In science, “pure” means only one substance present—the same kind of particle everywhere in the sample.
What Are the Types of Pure Substances?
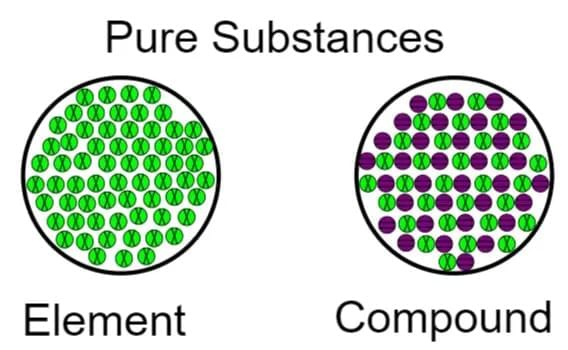
Pure substances can be divided mainly into: Elements & Compounds
 Physical Changes Do Not Change the Type of Pure Substance
Physical Changes Do Not Change the Type of Pure Substance
- When water changes from ice to liquid to vapor and back, it’s still water—the basic particles remain the same.
- A physical change (like melting or boiling) only changes state, not the type of substance.
Passing Electricity Through Water
- Electrolysis of water produces two different gases (not water vapor): one that makes a “pop” sound with a flame (hydrogen), the other that makes a flame glow brighter (oxygen).
- Water breaks down (chemically) into hydrogen and oxygen—proving it is a compound made of two elements.
Elements
Elements are pure substances made of only one type of atom.
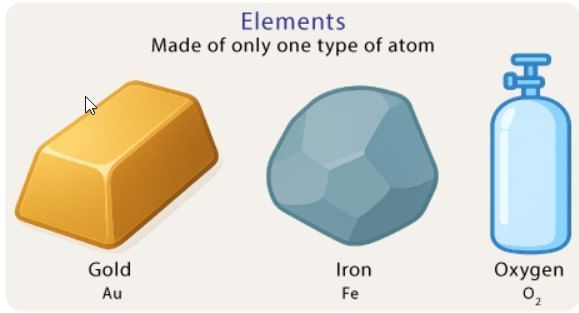
- Cannot be broken into simpler substances by chemical means.
- Each element has its own type of particles (atoms).
- Examples: Hydrogen, oxygen, gold, silver, sulfur, carbon.
Most elements exist as molecules (atoms grouped together):
- H₂ (hydrogen), O₂ (oxygen).
- Metals (gold, iron), non-metals (oxygen, sulfur), and metalloids (silicon).
Classification of Elements: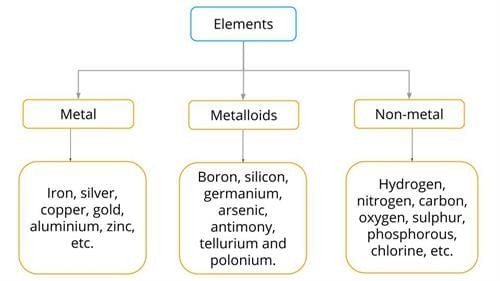
Metals: E.g., gold, silver, magnesium, iron, aluminum (good conductors, shiny, malleable).
Non-metals: E.g., carbon, sulfur, hydrogen, oxygen (poor conductors, brittle).
Metalloids: Elements with properties between metals and non-metals, e.g., silicon, boron (learned in higher grades).
Key Facts:
- Total known elements: 118.
- Most are solids at room temperature.
- 11 are gases (all non-metals, e.g., oxygen, helium, nitrogen).
- 2 are liquids: Mercury (metal) and bromine (non-metal).
- Special cases: Gallium and caesium (solids) melt to liquids around 30°C (303 K).
Compounds
Compounds are pure substances made by the chemical combination of two or more elements in a fixed ratio.
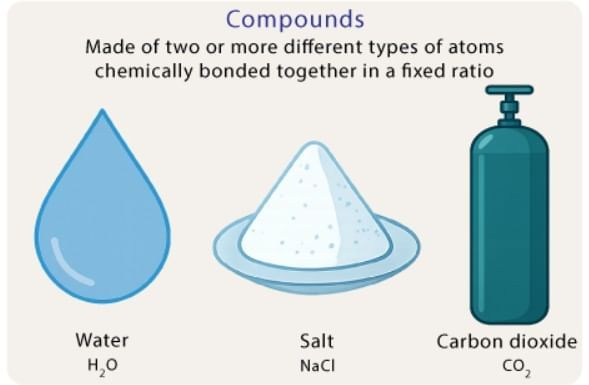
Key Properties:
- Formed in fixed ratios (e.g., water: 2 hydrogen atoms to 1 oxygen atom, ratio 2:1).
- New substance with unique properties (e.g., hydrogen is a fuel, oxygen supports burning, but water extinguishes fire).
Examples:
- Water: Compound of hydrogen and oxygen.
- Sodium Chloride (Common Salt): Compound of sodium (soft metal) and chlorine (hazardous gas) in 1:1 ratio. Harmless and used for taste; can be separated from water by evaporation (physical), but sodium and chlorine require chemical separation.
- Sugar: Compound of carbon, hydrogen, and oxygen (decomposes to carbon and water on heating).
Intersting Fact: Mobile phones use over 45 elements like aluminum, copper, silicon, cobalt, lithium, gold, silver for screens, batteries, etc.
Mixture vs. Compound - Iron and Sulfur
In this experiment, we explore how iron and sulfur behave as a mixture before heating and form a compound after heating. This highlights key differences between mixtures (where substances stay separate) and compounds (where they combine chemically into something new). Let's break it down step by step, starting with the initial mixture.
Before Heating: Forming Sample A (The Mixture)
To begin, mix iron filings and sulfur powder together to create Sample A. This is a classic example of a mixture, where the two substances are simply combined without any chemical change.
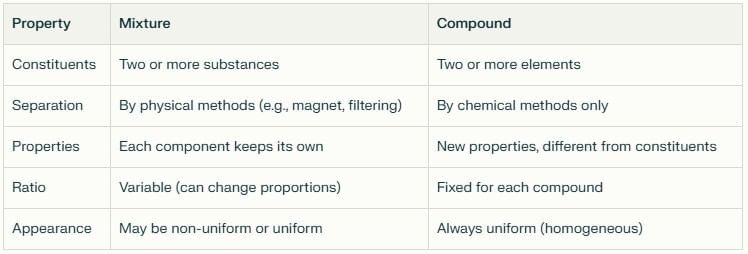
Appearance: You can clearly see both components as separate substances—black iron filings and yellow sulfur powder—making it look non-uniform.
Magnet Test: When you bring a magnet near Sample A, it attracts only the iron filings, leaving the sulfur behind. This shows the components retain their individual properties.
Acid Test: Add dilute hydrochloric acid to Sample A. The iron reacts to produce hydrogen gas (which makes a "pop" sound when ignited), while the sulfur does not react and remains as a yellow solid.
These tests confirm that in a mixture, substances can be separated easily and keep their original traits. Now, let's see what happens when we apply heat to transform this mixture.
After Heating: Forming Sample B (The Compound)
Next, gently heat Sample A while stirring. This causes a chemical reaction, resulting in a new black mass called iron sulfide (Sample B). The transformation shows how elements combine to form a compound with entirely new properties.
Appearance: The black mass looks uniform throughout—no separate iron or sulfur visible anymore.
Magnet Test: Unlike Sample A, a magnet has no effect on Sample B. The iron is now chemically bound and doesn't respond magnetically.
Acid Test: Add dilute hydrochloric acid to Sample B. It produces hydrogen sulfide gas, which has a distinct rotten egg smell—completely different from the odorless hydrogen gas in Sample A.
At this point, iron and sulfur can no longer be separated by physical methods like magnets or simple filtering. A compound has formed, with fixed ratios and unique characteristics that differ from the original elements.
Differences Between Mixtures and Compounds
How Do We Use Elements, Compounds, and Mixtures?
Elements are basic building blocks, compounds create new substances with unique traits, and mixtures combine properties for practical uses. This knowledge drives innovation in health, food, construction, and environmental protection—showing how science improves daily life.
Everyday Presence of Elements, Compounds, and Mixtures
- Elements, compounds, and mixtures surround us: They form the basis of everything we see and use.
- Air: A mixture of gases like oxygen, nitrogen, and carbon dioxide—essential for breathing.
- Water: A compound made from elements hydrogen and oxygen—vital for life.
- Metals for construction: Elements like iron and aluminum are used to build bridges, buildings, and vehicles due to their strength and durability.
Role in Innovation and Science
- Key to new discoveries: Understanding how elements combine into compounds helps create innovative products.
- Medicines and vaccines: Chemists study element combinations to develop life-saving drugs that fight diseases.
- Fertilizers for agriculture: Knowledge of compounds enhances crop production, helping feed the growing global population.
- Engineering and materials: Engineers use compounds and mixtures to design stronger materials. Example: Alloys like stainless steel (a mixture) are stronger and more durable than pure iron.
Building Materials as Mixtures
- Common construction items are often mixtures: Wood, steel, and concrete—all mixtures used for their combined properties like strength and flexibility.
- Minerals and metals: Many metals (elements) are extracted from minerals, which are naturally occurring mixtures or compounds.
Special Example: Graphene Aerogel (A 'Wonder' Material) Graphene Aerogel
Graphene Aerogel
What is it?: A lightweight material made from carbon (an element); known as the lightest material on Earth—so light that even grass can hold it.
Properties: Highly porous (full of tiny holes), giving it excellent absorbing capacity.
Uses:
Environmental cleanup: Absorbs oil spills in seas and on land, acting as a cleaner.
Energy and construction: Useful for energy-saving devices and special coatings for buildings to improve efficiency.
What Are Minerals?
Minerals are Naturally occurring substances found in rocks; most rocks are a mixture of different minerals.
Viewing Minerals: Can be seen with the naked eye, a magnifying glass, or a microscope.
Building Blocks: Minerals are either pure elements or compounds made of more than one element.
Relation to Matter: Elements and compounds in minerals are the basic building blocks of matter—anything that has mass and takes up space.
Types of Minerals: Native and Compound
1. Native Minerals: Pure elements (not compounds); occur naturally in their elemental form.
Metals: Examples include gold, silver, copper.
Non-metals: Examples include sulfur, carbon.
2. Compound Minerals: Most common type; made up of two or more elements combined chemically.
Examples: Quartz, calcite, mica, pyroxene, olivine, talc.
Everyday Uses of Minerals
Minerals (or elements extracted from them) are used in many daily items due to their properties.
Examples:
Cement: Made from minerals like calcite, quartz, alumina, and iron oxide (or obtained from minerals); used in construction.
Talcum Powder: Made from the mineral talc; used for personal care.
Minerals provide raw materials for buildings, tools, and products, showing how elements and compounds from nature support human needs.
What Is Matter? (Concluding Ideas)
Matter: Everything that has mass and takes up space; built from elements and compounds (e.g., rocks, water, air). Examples of Matter: Materials we see and use daily, like minerals, metals, and mixtures.
Non-Matter: Not everything is matter—some things lack mass or volume. Examples: Light, heat, electricity, thoughts, and emotions; these are important but not made of particles like elements or compounds.
Why It Matters: Understanding matter vs. non-matter helps us better grasp the world, from science to innovation.
|
54 videos|234 docs|13 tests
|
FAQs on Nature of Matter: Elements, Compounds, and Mixtures Chapter Notes - Science Curiosity Class 8 - New NCERT
| 1. What is the difference between a mixture and a pure substance? |  |
| 2. What are the two main types of pure substances? |  |
| 3. How do we use elements, compounds, and mixtures in daily life? |  |
| 4. What is a mineral, and how does it relate to pure substances? |  |
| 5. Can mixtures be separated, and if so, how? |  |





















