Matter Chapter Notes | Science for Grade 7 PDF Download
| Table of contents |

|
| Introduction |

|
| Matter |

|
| States of Matter |

|
| Changes in State of Matter |

|
| Points To Remember |

|
| Glossary |

|
Introduction
In this chapter, we will explore the world of matter, which is everything around us that we can see, touch, and feel. Matter is what makes up all the things in our surroundings, like our books, toys, water, and even the air we breathe. We will learn about the tiny building blocks of matter called molecules and how they behave in different forms, such as solids, liquids, and gases. Through simple activities, we will discover the properties of matter and how it can change from one form to another, like when ice melts into water or water turns into steam when heated.
Matter
- Matter is anything with mass and volume, including trees, rocks, vehicles, humans, animals, and food.
- The entire universe consists of matter in various forms.
- Matter is composed of elements in different proportions.
- An element is a substance that cannot be broken down into simpler substances by physical or chemical means.
- An atom is the smallest unit of an element, which may or may not exist independently but always participates in chemical reactions.
- Even the basic unit of the human body, the cell, is made of elements like hydrogen, carbon, oxygen, and phosphorus, which are themselves composed of atoms.
Molecules
- Matter is made up of very tiny particles called molecules.
- These molecules can be made of the same kind of atoms, like in oxygen (O₂) and hydrogen (H₂).
- Some molecules are made of different kinds of atoms, like in water (H₂O) and carbon dioxide (CO₂).
- Molecules are so small that we cannot see them even with a powerful microscope.
- A scientist named John Dalton found that matter is made of tiny particles called molecules.
- Another scientist named Acharya Kanad, an Indian sage and philosopher, said that matter is made of tiny particles called "anu" (molecules) and even smaller particles called "paramanu" (atoms).
- Acharya Kanad also said that atoms can combine in different ways to form molecules when heated.
- A theory called the Kinetic Theory of Matter, made in the year 1860, says that matter is made of a large number of small particles called atoms, which are always moving.
- This theory helps us understand how matter behaves and how the tiny particles are arranged in different forms of matter.
Characteristics of Molecules
1. Molecules are very small in size:
- Molecules are very tiny particles.
- We cannot see them with our eyes because they are so small.
- The size of molecules is very small, about 10⁻⁸ to 10⁻¹⁰ meters.
Activity 1.1
Aim: To show that molecules have a very small size
Materials required: Three beakers (50 mL each), some crystals of potassium permanganate, water
Procedure:
- Take 3-4 crystals of potassium permanganate and mix them in 50 mL of water in a beaker.
- You will get a deep purple-colored solution. Mark this as beaker A.
- Take 10 mL of the purple solution from beaker A and add it to another beaker with 40 mL of water. The water will turn purple but lighter than before. Mark this as beaker B.
- Now, take 10 mL of the colored water from beaker B and add it to another beaker with 40 mL of water. The water will change color but will be even lighter. Mark this as beaker C.
Observation: Even though the solution gets lighter each time, the water still turns purple, showing that potassium permanganate is present.
Conclusion: This activity shows that there are millions of tiny particles of potassium permanganate in a small crystal, which spread out in the water. So, molecules are very small.
2. Molecules have spaces between them:
- Molecules in any material are arranged in a way that there is space between them.
- This space between molecules is called the intermolecular space.
- The space between molecules is different in solids, liquids, and gases.
- In solids, the space between molecules is very small.
- In liquids, the space between molecules is more than in solids.
- In gases, the space between molecules is the largest.
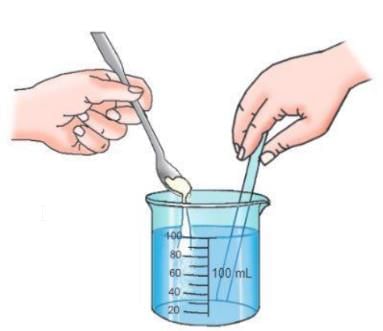
Activity 1.2
Aim: To demonstrate the presence of spaces between particles of matter
Materials required: A measuring cylinder (100 mL), a glass rod, powdered sugar, a spoon, water
Procedure:
- Take 100 mL of water in a measuring cylinder and mark the level of water.
- Dissolve some powdered sugar in the water with the help of a glass rod.
Observation: Even though the sugar has dissolved in the water, the water level stays the same as before.
Conclusion: This activity shows that when sugar is dissolved in water, the sugar particles go into the spaces between the water particles. This means there is space between the molecules of water. So, molecules of matter have spaces between them.
3. Molecules are constantly moving:
- Molecules are always moving and never stop.
- They move in a zigzag way, not in a straight path.
- This zigzag movement is called random motion.
- For example, when we spray perfume in one corner of a room, the smell spreads everywhere because the perfume molecules move in a zigzag way.
- Another example is when we burn an incense stick; the smell spreads because the molecules of the incense move in a zigzag way.
- Take a beaker with clear water and add a drop of blue ink into it.
- You will see that the blue color of the ink slowly spreads in the water, and soon the whole beaker looks light blue.
- This spreading of ink in water is called diffusion.
- Diffusion happens because the molecules of ink and water are always moving.
4. Molecules attract each other:
- Molecules always pull each other closer because they have a force of attraction between them.
- This force of attraction between molecules is called the intermolecular force of attraction.
- The force of attraction is different for different materials, which is why materials have different properties.
- For example, some materials are hard like rocks, some are in small crystals like sugar, and some are in powdered form.
Activity 1.3
Aim: To demonstrate the intermolecular force of attraction in different substances
Materials required: A piece of chalk, a piece of coal, an iron nail, a hammer
Procedure: Take a piece of chalk, a piece of coal, and an iron nail. Beat them with a hammer.
Observation: The piece of chalk breaks easily compared to the piece of coal. The iron nail is very hard to break.
Conclusion: This activity shows that different substances have different forces of attraction between their molecules. The force of attraction in chalk is the least, so it breaks easily. The force of attraction in iron is very strong, so it is hard to break.
The intermolecular force of attraction of two types:
- The force of cohesion: The force of attraction between the same kind of molecules is called the force of cohesion. For example, the force of attraction between water molecules, mercury, wood, glass, etc., is the force of cohesion.
- The force of adhesion: The force of attraction between different kinds of molecules is called the force of adhesion. For example, when you apply glue between paper and glass, the glue sticks to both because of the force of adhesion. Another example is when water drops stick to a glass surface; this happens because of the force of adhesion between water and glass.
States of Matter
- At normal temperature, matter is found in three forms: solids, liquids, and gases.
- There is another state called plasma, which is found at very high temperatures, like in Bose-Einstein condensates.
- Another state called Bose-Einstein condensate is found at very low temperatures.
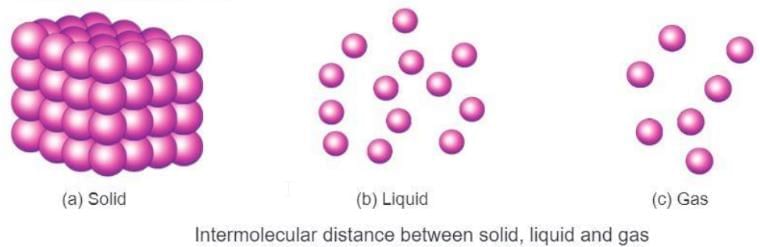
- In solids, liquids, and gases, the way molecules are arranged, the space between them, and the force of attraction between them are different.
Let’s learn about the properties of solids, liquids, and gases:
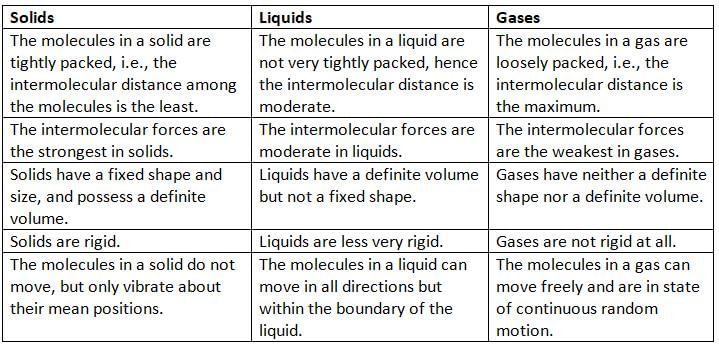
Solids
- Solids are a form of matter where the molecules are packed very closely together.
- The space between the molecules in solids is very small.
- The force of attraction between the molecules in solids is very strong.
- Solids have a fixed shape and size, like chalk or wood.
- Solids have a fixed volume.
- The molecules in solids do not move freely; they only vibrate in their positions.
- Solids cannot be compressed easily when pressed.
- When heated, solids expand very little because the force of attraction between the molecules is very strong.
- Solids are hard and rigid, but some can be beaten into sheets or turned into powder.
Liquids
- Liquids are a form of matter where the molecules are not very closely packed.
- The space between the molecules in liquids, like water or mercury, is more than in solids.
- The force of attraction between the molecules in liquids is weaker than in solids, so they are loosely packed.
- The molecules in liquids are not fixed in one place and can move freely.
- Liquids do not have a fixed shape; they take the shape of the container they are poured into.
- Liquids have a fixed volume.
- Even though the force of attraction in liquids is weaker than in solids, the molecules are not completely free.
- Liquids are almost incompressible, which means they cannot be pressed easily.
- When heated, liquids expand because the force of attraction between the molecules is weak.
- Liquids are less rigid than solids.
Gases
- Gases are a form of matter where the molecules are very loosely packed.
- The space between the molecules in gases, like air or oxygen, is very large.
- The force of attraction between the molecules in gases is very weak, so the molecules are very loosely packed.
- The molecules in gases can move freely in all directions in a zigzag way.
- Gases do not have a fixed shape or volume; they take the shape and volume of the container they are in.
- Gases can be compressed easily into a smaller space.
- Gases expand a lot when heated because the force of attraction between the molecules is very weak.
- Gases are not rigid at all.
Tips: Burning garbage creates harmful gases that can make the air dirty and cause many diseases. Dirty air spreads quickly in the space around us because the molecules in gases move fast. It is better not to burn garbage in the open; instead, we should use proper ways to manage garbage.
Differences between Solids, Liquids, and Gases
Changes in State of Matter
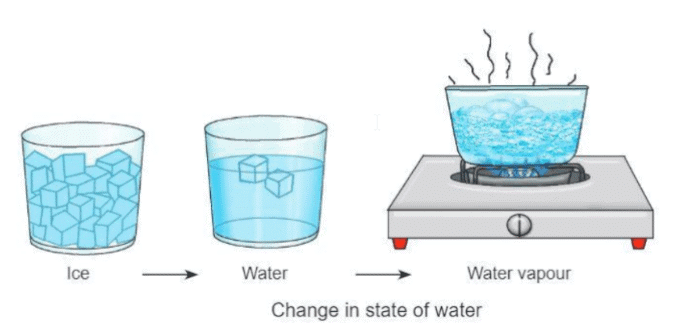
- Matter can change from one form to another, like from solid to liquid or liquid to gas.
- For example, water can be in three forms: ice (solid), water (liquid), and water vapor (gas).
- When ice is heated, it melts and turns into water (liquid).
- When water is heated in a pan, it turns into water vapor (gas).
- When water vapor cools down, it turns back into water (liquid), like when ice on a pan freezes.
- These changes happen through processes like melting, boiling, evaporation, and condensation.
Points To Remember
- Matter is anything that has weight and takes up space. Matter is made of different elements mixed in different amounts.
- A molecule is the smallest unit of matter that can exist on its own and keep all the physical and chemical properties of that matter.
- Molecules of matter are very small in size, have spaces between them, are always moving, and attract each other.
- Matter exists in three forms: solid, liquid, and gas, based on how the molecules are arranged, the space between them, and the force of attraction between them.
- In solids, the molecules are very closely packed. In liquids, the molecules are not very closely packed. In gases, the molecules are very loosely packed.
- Matter can change from one form to another, like from solid to liquid or liquid to gas.
Glossary
- Matter: Anything that has weight and takes up space.
- Element: A substance that cannot be broken into two or more simpler substances by any physical or chemical method.
- Atom: The smallest unit of an element that may or may not exist on its own but always takes part in a chemical reaction.
- Molecule: The smallest unit of matter that can exist on its own and keep all the physical and chemical properties of that substance.
- Intermolecular distance: The distance between the molecules of a substance.
- Intermolecular force: The force of attraction between the molecules of a material.
- Solid: The state of matter in which the molecules are very closely packed.
- Liquid: The state of matter in which the molecules are not very closely packed.
- Gas: The state of matter in which the molecules are very loosely packed.
|
87 videos|170 docs|69 tests
|
FAQs on Matter Chapter Notes - Science for Grade 7
| 1. What is matter and what are its main properties? |  |
| 2. What are the different states of matter? |  |
| 3. How do changes in temperature affect the state of matter? |  |
| 4. What is the difference between mass and weight? |  |
| 5. Can matter change its state? If so, how? |  |