Language of Chemistry Chapter Notes | Chemistry Class 8 ICSE PDF Download
Introduction
In this chapter, we will learn about the basics of chemistry language. Chemistry uses special symbols and formulas to show elements, compounds, and reactions. We will understand what elements are, how they are represented, and how they combine to form compounds. We will also learn about valency, which tells us how elements join together, and how to write chemical equations to show reactions. This chapter will help us understand how to balance equations and why reactions happen, using simple rules and examples.
Elements and Their Symbols
- An element is a pure substance made of only one type of atom.
- Each element has a special symbol to represent it, like O for oxygen.
- Symbols are decided by the International Union of Pure and Applied Chemistry (IUPAC).
- Symbols are usually short forms of the element's English, Latin, or Greek name.
- Rules for writing symbols of elements:
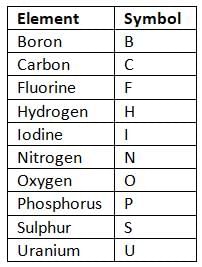
- Most element symbols come from the first letter of their English name, like N for nitrogen and C for carbon.
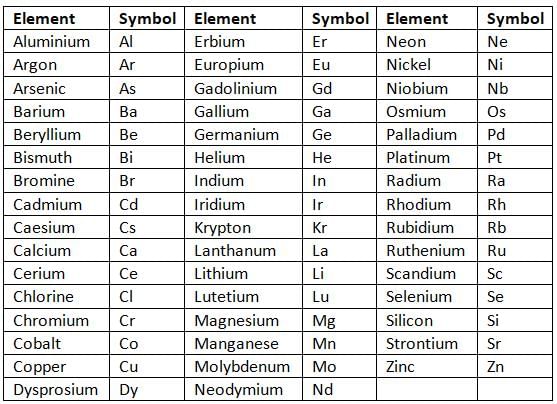
- If two elements start with the same letter, the symbol uses two letters, like Cl for chlorine (carbon is already C).
- When using two letters, the first letter is capital, and the second is small, like Cl for chlorine.
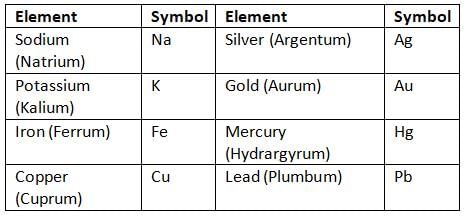
- Some symbols come from Latin, Greek, or German names, like Na for sodium (from Latin name natrium).
Did You Know?
Jöns Jacob Berzelius (1779-1848) was the first scientist to use letters as symbols for elements. We still use his method today.
Symbols Derived from the First Letter of the Names of Elements
Symbols Derived from Two Letters of the Names of Elements
Symbols Derived from Latin Names of Elements
Compounds and Their Formulae
A compound is a substance made when two or more elements join together in a fixed ratio. A chemical formula shows the elements in a compound and how many atoms of each element are present. For example, CO₂ (carbon dioxide) has 1 carbon atom and 2 oxygen atoms.
Valency
Valency of an element means its ability to combine with other atoms. It shows how many atoms of the element can join in a compound. Valency is always a whole number and usually ranges from 1 to 8.
Valency of Elements
- The outermost shell of an atom is called the valence shell.
- Electrons in the valence shell are called valence electrons.
- Valency is the number of electrons an atom can gain, lose, or share to form a compound.
- Valency is usually equal to the number of valence electrons if the atom has 4 or fewer electrons in the outermost shell.
- If an atom has more than 4 valence electrons, the valency is 8 minus the number of valence electrons.
- For example, oxygen has 6 valence electrons, so its valency is 8 - 6 = 2.
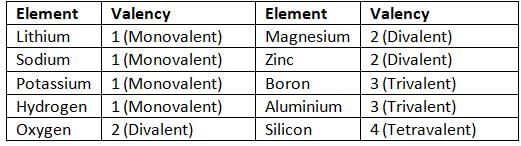
- Some elements have only one valency, like hydrogen (valency 1).
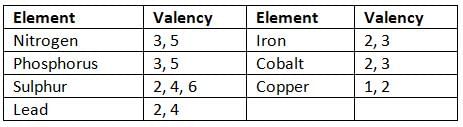
- Some elements have two valencies, like copper (valency 1 or 2).
- Some elements have more than two valencies, like iron (valency 2 or 3).
Elements Exhibiting Single Valency
Elements Exhibiting Variable Valency
Valency of an Element or Radical
Valency of an element or radical is the number of hydrogen atoms it can combine with or replace.
- Example: In HCl, 1 chlorine atom combines with 1 hydrogen atom, so chlorine's valency is 1.
- In H₂O, 1 oxygen atom combines with 2 hydrogen atoms, so oxygen's valency is 2.
- In NH₃, 1 nitrogen atom combines with 3 hydrogen atoms, so nitrogen's valency is 3.
- In CH₄, 1 carbon atom combines with 4 hydrogen atoms, so carbon's valency is 4.
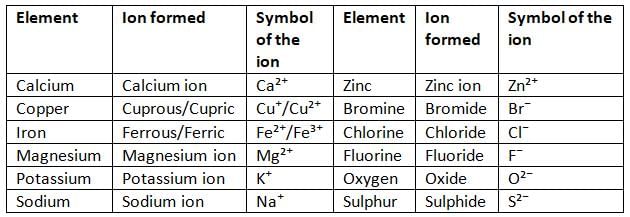
Valency of Ions
- An ion is a charged particle formed when an atom or group of atoms gains or loses electrons.
- A cation is an ion with a positive charge, formed when an atom loses electrons.
- An anion is an ion with a negative charge, formed when an atom gains electrons.
Elements, Ions Formed and their Symbols
Radicals
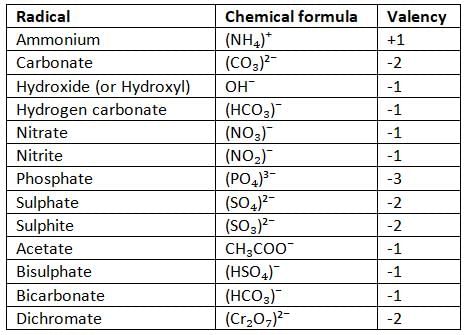
A radical is a group of different atoms that act as a single unit with a positive or negative charge.
Radicals, their Formulae and their Valency
Chemical Formulae of Compounds
A chemical formula shows the elements in a compound and the number of atoms of each element.
Steps to write a chemical formula:
- Step 1: Write the symbols of the elements or radicals in the compound.
- Step 2: Write the valency of each element or radical below the symbols.
- Step 3: Interchange the valencies and write them as subscripts next to the symbols.
- Step 4: Simplify the formula by dividing the subscripts by a common number if possible.
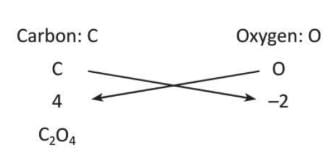
Let's understand the representation of compounds with the help of example:
Example: A molecule of carbon dioxide is formed by combining the atoms of elements carbon and oxygen.
Simplify the formula by dividing the valencies by common factor 2.
C02
Therefore, the chemical formula of carbon dioxide is C02.
Significance of a Chemical Formula
The chemical formula of a compound indicates the following information:
- The name of the compound.
- The names of the constituent elements.
- The number Of atoms Of various elements or ions present in one molecule Of the compound.
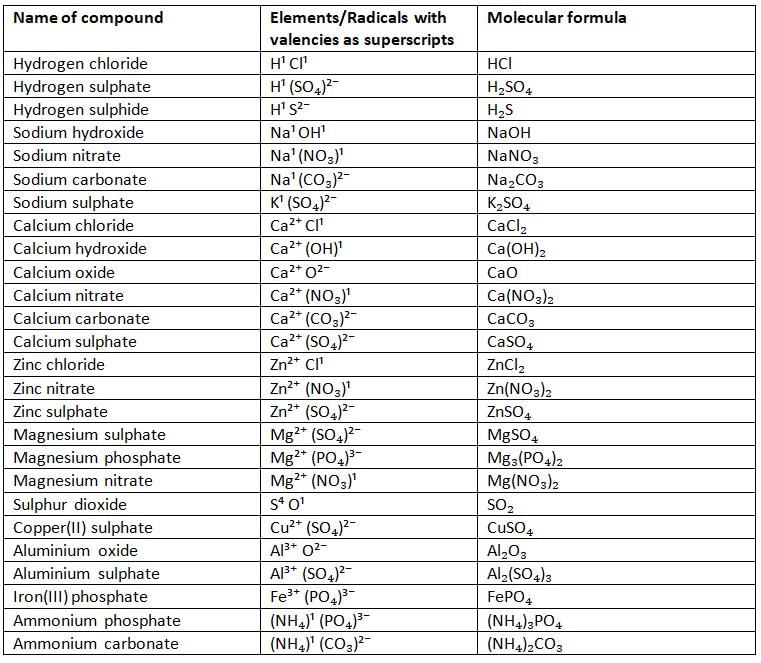
Molecular Formulae of Some Common Compounds
Chemical Equations (From Word Equations)
- A symbol is used to show an element, and a chemical formula is used to show a compound.
- A chemical equation shows a chemical reaction using symbols and formulas instead of words.
- A word equation shows the chemical reaction using the names of substances involved.
- For example: Sodium + Chlorine → Sodium chloride (this is a word equation).
- The same reaction can be written using symbols as: Na + Cl → NaCl (this is a chemical equation).
Steps to Write a Chemical Equation
- Reactants are always written on the left-hand side with a plus sign (+) between them.
- Products are always written on the right-hand side with a plus sign (+) between them.
- An arrow (→) is placed between reactants and products to show the direction of the reaction.
- In some reactions, a reversible arrow (⇌) is used to show the reaction can go in both directions.
- The physical state of reactants and products is shown using symbols:
- (s) is used for solid state.
- (l) is used for liquid state.
- (aq) is used for aqueous solution, which means dissolved in water.
- (g) is used for gaseous state.
- Any gas that is produced is shown with an upward arrow (↑).
- Any precipitate (solid that forms and settles) is shown with a downward arrow (↓).
- An exothermic reaction (which gives out heat) is shown by writing "+ Heat" on the product side (right-hand side).
- An endothermic reaction (which takes in heat) is shown by writing "+ Heat" on the reactant side (left-hand side).
- Sometimes, pressure, temperature, or a catalyst (a substance that speeds up the reaction) is written above or below the arrow.
Based on the Above-Mentioned Steps, Let’s Write Down the Chemical Equations for Some Reactions
- Reaction 1: Hydrogen combines with oxygen to form water.
- Word equation: Hydrogen + Oxygen → Water
- Chemical equation: H₂ + O₂ → H₂O
- Reaction 2: Methane burns in air to form carbon dioxide and water.
- Word equation: Methane + Oxygen → Carbon dioxide + Water
- Chemical equation: CH₄ + O₂ → CO₂ + H₂O
Note About Unbalanced Equations
- In the first reaction (H₂ + O₂ → H₂O), the number of hydrogen and oxygen atoms on the left-hand side and right-hand side are not equal.
- In the second reaction (CH₄ + O₂ → CO₂ + H₂O), the number of hydrogen, oxygen, and carbon atoms on the left-hand side and right-hand side are not equal.
- When the number of atoms of reactants and products is not equal, the equation is called a skeletal or unbalanced equation.
The Law of Conservation of Mass
- The law of conservation of mass says that matter cannot be created or destroyed in a chemical reaction.
- This means the total mass of reactants must be equal to the total mass of products.
- In other words, the total number of atoms of reactants must be equal to the total number of atoms of products.
Need for Balancing a Chemical Equation
- A skeletal equation has an unequal number of atoms of elements in reactants and products.
- The law of conservation of mass says matter cannot be created or destroyed in a reaction.
- So, the number of atoms of each element in reactants must equal the number in products.
- A balanced equation has an equal number of atoms of each element on both sides.
Balancing a Chemical Equation
We balance equations using the atomic theory and the law of conservation of mass.
Applying Atomic Theory
According to atomic theory, atoms in the reacting molecules do not get destroyed or changed during a chemical reaction. Instead, they either share or transfer electrons to create new compounds. This means that the number of atoms for each element must remain the same on both sides of a chemical equation. Based on this idea, chemical equations are balanced. Unbalanced or skeletal equations are balanced using the hit and trial method.
Let's look at an example to see how a chemical equation is balanced:
Example: Methane burns in air to form carbon dioxide and water.
Count the number of atoms in the reactants and the products.
The number Of carbon atoms are equal on both the sides but the number Of hydrogen and oxygen atoms are not equal. There are 4 hydrogen atoms on the left-hand side but only 2 hydrogen atoms on the right-hand side. In order to have 4 hydrogen atoms on the right-hand side, we have to multiply H20 by 2.
Now there are 2 oxygen atoms on the left-hand side and 4 oxygen atoms on the right-hand side. In order to have 4 oxygen atoms on the left-hand side, we have to multiply 02 by 2. By doing so, the equation changes to the following.
Again count the number of atoms on both the sides.
The chemical equation, CH4 + 202 → C02 + 2H20, now contains an equal number of atoms in the reactants and products. Hence, it is a balanced equation.
Applying the Law of Conservation of Mass
The law of conservation of mass says the total mass of reactants equals the total mass of products. We can use atomic masses to check if an equation follows this law:
Example: Balance the following equation.
Mg + O₂ → MgO
Solution:
The above equation is not balanced as the number of various atoms on both the sides is not equal.The balanced equation for the equation can be written as shown below.
2Mg + O₂ → 2MgOThe atomic masses of all the elements are shown below.
Mg = 24 u, O = 16 uBy using these atomic masses, we can find the atomic masses of the compounds involved in the reaction.
2Mg = 2 × 24 u = 48 u
O₂ = 2 × 16 u = 32 u
2MgO = 2 × (24 + 16) = 2 × 40 u = 80 uTotal mass of the reactants on LHS = 48 + 32 = 80 u
Total mass of the products on RHS = 80 uTherefore, the total mass of the reactants is equal to the total mass of the products which conforms to the law of conservation of mass.
Therefore, the balanced equation is:
2Mg + O₂ → 2MgO
Information Gathered from a Chemical Equation
A chemical equation provides us with only limited information about the chemical reaction it represents.
Let’s take an example of the following equation to know about the information it provides:
CaCO₃ (Calcium carbonate) → CaO (Calcium oxide) + CO₂ (Carbon dioxide)
The above equation tells us about the following things:
- It tells us about the name of the reactant(s).
Reactant: CaCO₃ - It tells us about the name of the product(s).
Products: CaO and CO₂ - It tells us about the number of molecules of each reactant participating in the reaction.
1 molecule of calcium carbonate - It tells us about the number of molecules of each product participating in the reaction.
1 molecule of calcium oxide and 1 molecule of carbon dioxide - It tells us about the proportion of reactants.
Here, we have only one reactant. - It tells us about the proportion of products.
Calcium oxide and carbon dioxide are in the ratio 1 : 1. - It tells us about the mass of the reactant(s).
The mass of calcium carbonate is 100 u. - It tells us about the mass of the products.
The mass of calcium oxide is 56 u and the mass of carbon dioxide is 44 u.
Limitations of a Chemical Equation
A chemical equation does not tell us everything about a reaction. We need to add more information to the equation.
- Physical States of Reactants and Products: Equations should show the physical state: (s) for solid, (l) for liquid, (g) for gas, (aq) for aqueous (in water).
- Pure State or Solution: Equations should show if a substance is pure or in solution, like silver nitrate in solid (s) or solution (aq).
- Heat and Some Temperatures: Some reactions need high or low temperatures; equations should show this.
- Light: Some reactions, like photosynthesis in plants, need light; equations should show this.
- Electricity: Some reactions, like breaking water into hydrogen and oxygen, need electricity; equations should show this.
- Pressure: Some reactions with gases need specific pressure; equations should show this.
- Catalyst: A catalyst speeds up a reaction without changing itself; equations should show the catalyst.
- Reversibility: Some reactions can go in both directions (reversible); equations should show this with a double arrow (⇌).
- Endothermic or Exothermic: Endothermic reactions absorb heat; exothermic reactions release heat; equations should show this by adding "+ Heat" on the correct side.
Points To Remember
- An element is the simplest form of a pure substance which is made up of only one kind of atom and is represented by a symbol.
- Each element is represented using a symbol, usually the short forms or abbreviated names of elements.
- A compound is represented using a chemical formula which represents the number of the elements that a molecule of a compound consists of.
- Valency is the combining capacity of an element. The valency of an element always is a whole number that varies from 1 to 8.
- An ion is formed when an atom or a group of atoms gains or loses electrons. A cation is a positively charged ion and an anion is a negatively charged ion.
- A radical refers to a group of different atoms present as a single unit having a positive or negative charge on it.
- A molecule of a compound is represented using a chemical formula.
- A word equation represents the chemical reaction using only the names of the reactants involved and products formed.
- The atomic theory states that atoms of the reacting molecules involved in a chemical reaction do not change.
- The law of conservation of mass states that matter is neither created nor destroyed in a chemical reaction. This law can be applied to the atomic masses of reactants and products to balance the chemical equation.
Glossary
- Element: The simplest form of a pure substance which is made up of only one kind of atom.
- Compound: A pure substance which is formed when two or more elements chemically combine in a definite proportion by mass.
- Valency: The combining capacity of an atom of an element.
- Valence shell: The outermost shell of an atom.
- Valence electrons: The electrons present in the valence shell.
- Ion: A charged particle which is formed when an atom or a group of atoms gains or loses electrons.
- Cation: An ion formed when an atom or a group of atoms loses electron(s) by developing a positive charge.
- Anion: An ion formed when an atom or a group of atoms accepts electron(s) by developing a negative charge.
- Radical: A group of different atoms present as a single unit having a positive or negative charge on it.
|
9 videos|46 docs|9 tests
|
FAQs on Language of Chemistry Chapter Notes - Chemistry Class 8 ICSE
| 1. What are the basic elements and their symbols in chemistry? |  |
| 2. How do you write the formula for a compound? |  |
| 3. What is a chemical equation, and how is it formed from word equations? |  |
| 4. Why is it important to balance a chemical equation? |  |
| 5. What limitations exist in representing chemical reactions with equations? |  |