Heat Transfer Chapter Notes | Food & Nutrition for Year 6 PDF Download
Introduction
Heat is a form of energy that makes things warm or cold. In this chapter, we will learn how heat moves and affects different things around us. We will study how heat changes the temperature, state, and size of objects. We will also explore how heat travels between objects when they touch each other, and how liquids turn into gas through processes like evaporation and boiling. Understanding heat transfer helps us know why things feel hot or cold and how we can use heat in our daily lives.
Heat Transfer
- Matter is made of tiny particles called molecules, which are very small, about 10^-10 meters in size.
- We cannot see these molecules even with a microscope because they are so tiny.
- Molecules in matter move all the time because they have energy called kinetic energy.
- When a substance gets heat, its molecules move faster, increasing their kinetic energy.
- When a substance loses heat, its molecules slow down, decreasing their kinetic energy.
- The total kinetic energy of all the molecules in a substance is called its internal kinetic energy.
- The total potential energy of all the molecules in a substance is called its internal potential energy.
- Internal kinetic energy plus internal potential energy together is called the internal energy of a substance.
- Heat is the internal energy of a substance, measured in joules (symbol: J).
- Heat flows from a hotter object to a colder object when two objects are in contact.
- The average kinetic energy of a substance decides its temperature.
- If the average kinetic energy of a substance increases, its temperature rises.
- If the average kinetic energy of a substance decreases, its temperature falls.
Note: 1 calorie = 4.2 joules (nearly)
Effects of Heat
Heat causes three main changes in a substance:
1. Change in Temperature of the Body
When a body gains heat, its temperature increases, and when it loses heat, its temperature decreases. The change in temperature depends on two things:
- Quantity of heat absorbed or released by the body:When a body absorbs heat, its temperature rises. When a body releases heat, its temperature falls.
- Reason: When a body is heated, its molecules move faster, so the average kinetic energy increases, and the temperature rises. When a body is cooled, its molecules move slower, so the average kinetic energy decreases, and the temperature falls.
- Material of the body: Some materials heat up to a high temperature even when a small amount of heat is given to them. Some materials need more heat to reach the same high temperature.
- Reason: Different materials need different amounts of heat to increase the temperature of the same mass by the same amount.
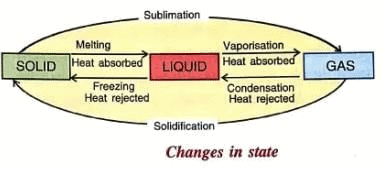
2. Change in State of the Body
- Matter exists in three states: solid, liquid, and gas.
- The change from one state to another at a constant temperature is called a change of state.
- When a solid is heated, it changes into a liquid at a fixed temperature, and this process is called melting.
- When a liquid is cooled, it changes into a solid at the same fixed temperature, and this process is called freezing or fusion.
- For example, ice melts into water at 0°C, and water freezes into ice at 0°C.
- When a liquid is heated, it changes into a gas at a fixed temperature, and this process is called vaporization or boiling.
- When a gas is cooled, it changes into a liquid at the same fixed temperature, and this process is called condensation.
- For example, water boils into steam at 100°C, and steam condenses into water at 100°C.
- When a solid is heated and changes directly into a gas at a fixed temperature, this process is called sublimation.
- When a gas is cooled and changes directly into a solid, this process is called solidification or deposition.
- For example, camphor changes from solid to gas on heating and from gas to solid on cooling.
- The change of state from liquid to gas at all temperatures is called evaporation.
- Evaporation is different from boiling because boiling happens at a fixed temperature, while evaporation happens at all temperatures.
- The heat absorbed or released during a change of state is called latent heat because it does not cause a change in temperature.
- This heat changes the average potential energy of the molecules, not their average kinetic energy.
- Latent heat is specific for each substance because each substance needs a different amount of heat to change its state.
3. Change in Size of the Body
- When a body is heated, it expands, which means its size increases.
- When a body is cooled, it contracts, which means its size decreases.
- This change in size due to heating or cooling is called thermal expansion.
- Almost all solids, liquids, and gases expand when heated and contract when cooled.
- Reason: When a substance is heated, the molecules move faster, and the average distance between the molecules increases, so the size of the substance increases. When a substance is cooled, the molecules move slower, and the average distance between the molecules decreases, so the size of the substance decreases.
Effect of Temperature on Molecular Motion
- Molecules of a substance have kinetic energy because they are always moving.
- When the temperature increases, the molecules move faster, so their kinetic energy increases.
- When the temperature decreases, the molecules move slower, so their kinetic energy decreases.
- At very high temperatures, the molecular motion is very fast.
- At a very low temperature, the molecular motion becomes very slow.
- The lowest possible temperature is called absolute zero, which is 0 on the Kelvin scale.
- At absolute zero, the molecular motion completely stops, but this temperature cannot be reached.
- Temperatures below absolute zero are not possible.
Change of Liquid into Vapour State
A liquid can change into a vapour in two ways:
- By evaporation at all temperatures.
- By boiling at a fixed temperature.
1. Evaporation
- Evaporation is the process where a liquid changes into its vapour at all temperatures from its surface.
- For example, if you put a drop of ether on your palm, it disappears quickly, and your palm feels cold because of evaporation.
- Evaporation happens at all temperatures and only from the surface of the liquid.
Explanation of Evaporation on the Basis of Molecular Motions
- It is hard to break a solid into small pieces, but a liquid can easily be broken into small drops.
- The force of attraction between molecules in a liquid is weaker than in a solid but stronger than in a gas.
- The weaker force of attraction in a liquid means the liquid molecules are not held in fixed positions and can move around.
- While moving, the liquid molecules collide with each other.
- Some molecules near the surface of the liquid gain energy from collisions and move faster than others inside the liquid.
- The molecules near the surface that gain enough energy can overcome the attractive forces of other molecules and escape into the air as vapour.
- These escaped molecules form the vapour of the liquid.
- The process continues until all the liquid evaporates.
- The rate of evaporation depends on temperature, wind, surface area, and humidity.
Effect of Temperature on the Rate of Evaporation
- When the temperature of a liquid increases, the rate of evaporation increases.
- Reason:
- At higher temperatures, the molecules of the liquid gain more energy and move faster.
- More molecules then have enough energy to reach the surface and escape as vapour, increasing the rate of evaporation.
Effect of Blowing of Air on the Rate of Evaporation
- When air blows over the surface of a liquid, the rate of evaporation increases.
- Reason:
- The blowing air carries away the vapour molecules from the surface of the liquid.
- This allows more liquid molecules to escape from the surface, increasing the rate of evaporation.
Effect of Increase in Area of Surface on the Rate of Evaporation
- When the surface area of a liquid increases, the rate of evaporation increases.
- Reason: A larger surface area means more molecules are at the surface and can escape into the air, so the rate of evaporation increases.
Effect of Presence of Humidity
- In the presence of humidity, the rate of evaporation slows down.
- Reason: When the air is humid, it already has a lot of water vapour, so there is less space for more vapour molecules to escape from the liquid.
Cooling Produced During Evaporation
- Evaporation causes cooling of the liquid.
- Reason:
- When a liquid evaporates, the molecules at the surface take heat from the liquid and the surrounding area to change into vapour.
- If there is no external heat source, the liquid takes heat from its surroundings, like your hand, and cools down.
- For example, your palm feels cool after ether evaporates because the liquid takes heat from your palm.
2. Boiling
- Boiling is the process where a liquid changes into its vapour at a fixed temperature.
- The temperature at which a liquid boils and turns into vapour without further increase in temperature is called its boiling point.
- For example, water boils at 100°C and turns into steam.
- During boiling, bubbles form throughout the liquid, and the temperature does not rise further.
- At 100°C, water turns into steam, so the boiling point of water is 100°C.
Explanation of Boiling by Molecular Motion
- When a liquid is heated, its molecules gain energy and move faster.
- The increased motion causes the molecules to collide with each other more often.
- Some molecules gain enough energy to overcome the attractive forces of other molecules.
- These molecules then form bubbles of vapour inside the liquid, not just at the surface.
- The bubbles grow in size as more molecules join them and rise to the surface of the liquid.
- The bubbles burst at the surface, releasing the vapour into the air.
- This process is called boiling and happens throughout the liquid.
- During boiling, the temperature of the liquid does not increase further; it stays at the boiling point.
Effect of Pressure on the Boiling Point
- The boiling point of a liquid increases with an increase in pressure and decreases with a decrease in pressure.
- Reason:
- At the boiling point, the pressure exerted by the vapour of the liquid is equal to the atmospheric pressure around it.
- When the atmospheric pressure increases, the vapour pressure of the liquid must also increase to match it, so the boiling point increases.
- When the atmospheric pressure decreases, the vapour pressure needed is less, so the boiling point decreases.
- This is why water boils at a lower temperature in the mountains, where the atmospheric pressure is lower.
- For Example, In a pressure cooker, the pressure is higher, so water boils at a higher temperature, around 125°C, which helps cook food faster.
Difference Between Evaporation and Boiling
Evaporation and boiling are both processes where a liquid changes into a vapour, but they are different in the following ways:
- Evaporation happens at all temperatures, while boiling happens only at a fixed temperature called the boiling point of the liquid.
- Evaporation happens only from the surface of the liquid, while boiling happens throughout the entire mass of the liquid at the same time.
- In evaporation, some molecules near the surface gain energy from collisions and escape as vapour, while in boiling, molecules throughout the liquid gain enough energy to form bubbles and escape as vapour.
- In evaporation, the heat needed to change the liquid into vapour comes from the surroundings, while in boiling, heat is supplied externally at a fixed temperature.
- Evaporation is a slow process, while boiling is a fast process.
Thermal Expansion
- When a substance like a solid, liquid, or gas is heated, it gets bigger in size.
- When the substance cools down, it gets smaller in size.
- We can see this expansion in solids, liquids, and gases, but it happens differently in each.
- Exceptions of thermal expansion:
- Water does not expand when heated from 0°C to 4°C; instead, it shrinks.
- Silver iodide shrinks when heated from 80°C to 141°C.
- Silica also shrinks when heated below -80°C.
Did You Know?
- 1 kg of water at 100°C absorbs 2.26 × 106 J of heat to convert into steam at 100°C.
- 1 kg of water at 0°C absorbs 3.34 × 105 J of heat to convert into water at 0°C.
Thermal Expansion in Solids
- When a solid is heated, it gets bigger in all directions.
- The length, area, and volume of the solid all increase when heated.
- The increase in length is called linear expansion.
- The increase in area is called superficial expansion.
- The increase in volume is called cubical expansion.
Explanation of Thermal Motion
- When we heat a solid, the tiny particles inside it gain more energy.
- These particles start moving and vibrating more around their positions.
- This movement makes the particles move farther apart from each other.
- Because the particles are farther apart, the solid gets bigger in all directions.
Demonstration of Thermal Expansion of Solids
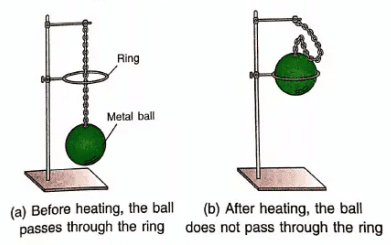
Experiment (1): Ball and Ring Experiment
- We use a metal ball and a metal ring for this experiment.
- At room temperature, the ball can just pass through the ring easily.
- When we heat the ball on a burner and then try to pass it through the ring, it does not pass.
- This happens because the ball gets bigger in size when heated and its diameter becomes larger than the ring's diameter.
- When the ball cools down, it becomes smaller again and can pass through the ring.
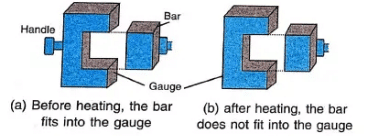
Experiment (2): Bar and Gauge Experiment
- We use a metal bar and a metal gauge for this experiment.
- At room temperature, the bar fits perfectly into the gauge.
- When we heat the bar and try to fit it into the gauge, it does not fit anymore.
- This happens because the bar gets longer when heated and becomes too big for the gauge.
- When the bar cools down, it becomes smaller again and fits into the gauge.
Linear Expansion
When a solid in the shape of a rod or wire is heated, it gets longer in length. This increase in length is called linear expansion.
The increase in length depends on three things:
- Dependence on the original length of the rod: If we heat two rods of the same material to the same temperature but one rod is longer than the other, the longer rod will expand more in length.
- Dependence on the increase in temperature: If we heat two rods of the same material and same length but heat one to a higher temperature than the other, the rod heated to the higher temperature will expand more in length.
- Dependence on the material of the rod:
- If we heat two rods of the same length to the same temperature but one is made of copper and the other of iron, the copper rod will expand more than the iron rod.
- The increase in length of the copper rod is about 1.5 times more than the iron rod.
Formula for linear expansion:
- If L₀ is the length of the rod at 0°C and Lₜ is the length at t°C, then the increase in length is: Lₜ - L₀ = L₀ α t.
- Here, α (alpha) is called the coefficient of linear expansion, and it is different for different materials.
- The unit of α is per °C.
Coefficient of Linear Expansion of Some Solids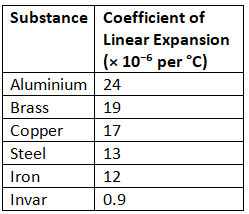
Note:
- Invar is an alloy of iron and nickel, which does not expand much on heating. This is why the pendulum of a clock is made of invar.
- Pyrex glass also expands very little on heating.
Did You Know?
- A bimetallic strip, made of two rods of equal length but different materials (like iron and copper) joined together, is often used in thermostats. Thermostats regulate temperature by opening or closing circuits and are found in electrical devices such as refrigerators, electric irons, ovens, geysers, and more.
- The expansion of a rod when heated does not depend on whether it is hollow or solid. If two rods of the same metal and length—one hollow and one solid—are heated to the same temperature increase, both will expand by the same amount.
Superficial Expansion of Solids
- When a metal plate is heated, its length and breadth both increase.
- This increase makes the area of the plate bigger, which is called superficial expansion.
- The increase in area depends on three things:
- The initial area of the plate: A larger initial area means more increase in area.
- The increase in temperature: A bigger rise in temperature means more increase in area.
- The material of the plate: A brass plate expands more than an iron plate for the same rise in temperature.
- Formula for superficial expansion:
- If A₀ is the area of the plate at 0°C and Aₜ is the area at t°C, then the increase in area is Aₜ - A₀ = A₀ β t.
- Here, β (beta) is called the coefficient of superficial expansion, and it is different for different solids.
- Relationship between α, β, and γ:
- The coefficients α (linear), β (superficial), and γ (cubical) are related as β = 2α and γ = 3α.
- So, α : β : γ = 1 : 2 : 3.
Cubical Expansion of Solids
- When a solid is heated, it expands in all directions—length, breadth, and thickness.
- This makes the volume of the solid increase, which is called cubical expansion.
- The increase in volume depends on three things:
- The initial volume of the solid: A larger initial volume means more increase in volume.
- The rise in temperature: A bigger rise in temperature means more increase in volume.
- The material of the solid: A brass ball increases in volume more than an iron ball of the same radius for the same rise in temperature.
- Formula for cubical expansion:
- If V₀ is the volume of a solid at 0°C and Vₜ is the volume at t°C, then the increase in volume is Vₜ - V₀ = V₀ γ t.
- Here, γ (gamma) is called the coefficient of cubical expansion, and it is different for different materials.
Some Applications of Thermal Expansion of Solids in Daily Life
1. Construction of a Bridge
- In bridges, steel girders are used.
- One end (A) of the girder is fixed in concrete, but the other end (B) is not fixed; it is placed on rollers.
- This is done so that if the temperature rises in summer or falls in winter, the girder can expand or contract without affecting the pillar or the bridge.
2. Railway Tracks
- Railway tracks are made of steel rails.
- Small gaps are left between the ends of two rails to allow for expansion in summer.
- If there were no gaps, the rails would expand in summer, touch each other, and bend sideways.
3. Riveting
- Riveting is used to join two steel plates together.
- Rivets are small steel rods that are heated until red-hot and then inserted into holes in the plates.
- The ends of the rivets are hammered to make heads while they are still hot.
- When the rivets cool down, they contract and pull the plates closer, making a strong joint.
- This method is also used in joining steel girders, boiler plates, etc.
4. Electric Cables and Telephone Wires
- Electric cables and telephone wires are placed between poles.
- In summer, the wires expand and sag because of the heat.
- In winter, the wires contract and become tight.
- To prevent the wires from breaking in winter due to contraction or sagging too much in summer due to expansion, they are kept slightly loose in summer and tight in winter.
5. Fitting the Steel Rim on a Horse Cart Wheel
- The wheel of a horse cart has a steel rim to make it strong and smooth.
- The steel rim is made slightly smaller in diameter than the wooden wheel.
- The rim is heated uniformly along its circumference until its diameter becomes slightly larger than the wooden wheel.
- The hot rim is then slipped over the wooden wheel.
- When the rim cools down, it contracts and fits tightly over the wooden wheel.
6. Glassware Used in Kitchen
- Kitchen glassware, like Pyrex glass, has a very low coefficient of cubical expansion.
- This means Pyrex glass expands very little when heated, so it does not crack easily.
7. Pendulum of a Clock is Made of Invar
- Invar is an alloy of iron and nickel.
- It has a very low coefficient of linear expansion (9 × 10⁻⁷ per °C).
- The pendulum of a clock is made of invar so that it does not expand much in summer or contract much in winter.
- This helps the clock keep accurate time.
8. Loosening a Glass Stopper or a Metal Screw Cap
- A glass stopper on a bottle can be loosened by warming the neck of the bottle.
- When the neck is warmed, it expands and gives space for the stopper to loosen.
- If there is a metal screw cap on the neck of a glass bottle, heating the screw cap makes it expand and loosen.
9. Cracking of a Thick Glass Tumbler
- A thick glass tumbler can crack when hot liquid is poured into it.
- Glass is a poor conductor of heat, so the inner surface of the tumbler gets hot and expands, while the outer surface stays at room temperature and does not expand.
- This unequal expansion causes the tumbler to crack.
Thermal Expansion in Liquids
- Liquids, like solids, get bigger when heated.
- Liquids expand a lot more than solids when heated.
- Liquids do not have a fixed shape, but they do have a fixed volume.
- So, when liquids expand, only their volume increases.
Exception
- Water is different from other liquids.
- When water is heated from 0°C to 4°C, it contracts (gets smaller).
- But when heated beyond 4°C, it expands (gets bigger).
- This strange behavior of water is called its anomalous behavior.
The cubic expansion of a liquid can be demonstrated by the following simple experiment
Experiment:
- Take an empty bottle with a tight cork that has a hole in the middle.
- Put a drinking straw, two bricks, a wire gauze, and a burner for the experiment.
- Fill the bottle completely with water mixed with a few drops of ink to make it colorful.
- Fix the cork tightly on the bottle and pass the drinking straw through the hole in the cork.
- Make sure some ink-water comes out of the straw to avoid air inside.
- Mark the level of the ink-water in the straw as the starting point.
- Place the bottle on the wire gauze, which is kept on the two bricks.
- Heat the bottle using the burner.
- Watch the level of ink-water in the straw after heating.
- You will see the level rises, which means the water has expanded due to heating.
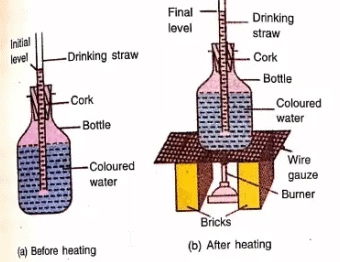
Explanation of thermal expansion of liquid by molecular motion
- In a liquid, the molecules (tiny particles) are free to move anywhere inside the liquid.
- When a liquid is heated, the kinetic energy (energy of movement) of the molecules increases.
- Because of more energy, the molecules move faster and more strongly.
- This makes the molecules push each other and move farther apart.
- When the molecules move apart, the liquid takes up more space, so it expands.
Factors affecting the cubic expansion of a liquid
The expansion of a liquid depends on three things:
- Original volume of the liquid: If the liquid has a larger volume to start with, it will expand more when heated.
- Rise in temperature: If the temperature increases by a larger amount, the liquid will expand more.
- Nature of liquid: Different liquids expand by different amounts when heated to the same temperature.
- For example, alcohol expands more than water for the same rise in temperature.
- If V₀ is the volume of a liquid at 0°C, and Vₜ is its volume at t°C,
- Then the increase in volume is Vₜ - V₀.
- So, Vₜ - V₀ = V₀ γ t, where γ is the coefficient of cubical expansion of the liquid.
Coefficient of cubical expansion of some common liquids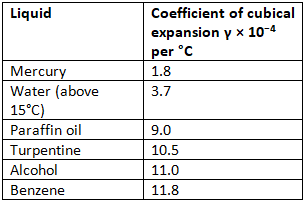
Application of thermal expansion of liquids in daily life
- Thermal expansion of liquids is used in the working of a mercury thermometer.
- We read the temperature using a thermometer that has a glass tube with mercury inside.
- The glass tube is connected to a small bulb at the end filled with mercury.
- When the bulb touches a hot body, the mercury expands because of the heat.
- The mercury rises in the glass tube and shows the temperature on the scale.
- The tube is marked to read the temperature, so we can measure how hot something is.
Did You Know?
When a liquid contained in a vessel is heated, the vessel first absorbs the heat and expands, causing the liquid's level to drop initially. As the heat reaches the liquid, it expands, and the liquid level rises. Since liquids expand more than solids, the liquid's level rises above its initial level. Therefore, the actual expansion of the liquid is greater than the observed expansion.
Thermal expansion in gases
- Gases also expand when heated, but they expand much more than liquids and solids.
- Unlike liquids, gases do not have a fixed shape or a fixed volume.
- So, gases only have cubical expansion (increase in volume) when heated.
Did You Know?
When a gas is heated, either its volume or pressure is kept constant. In both scenarios, the expansion effect is equivalent: there’s an increase in pressure if the volume is held constant, or an increase in volume if the pressure is held constant.
Explanation of thermal expansion in gases by molecular motion
- In a gas, the molecules (tiny particles) have more energy to move than in liquids or solids.
- When a gas is heated, the molecules gain even more energy and move faster.
- The faster-moving molecules hit each other and the walls of the container more often.
- This causes the gas to take up more space, so the gas expands.
Variation of Density with Temperature
- When a substance (like a liquid or gas) is heated, its volume increases.
- But the mass of the substance stays the same because no material is added or removed.
- Since density is mass divided by volume (density = mass/volume), the density decreases when the volume increases.
- In solids, when the temperature increases, the volume increases a little, so the density decreases a little.
- In liquids and gases, the volume increases a lot with temperature, so the density decreases a lot.
Exception
- Water is special because it contracts (gets smaller) when heated from 0°C to 4°C.
- So, the density of water increases from 0°C to 4°C because the volume decreases.
- At 4°C, water has its maximum density, which is 1000 kg/m³.
- Above 4°C, water expands when heated, so its density decreases.
Do you know?
If an iron washer is heated, its mass will remain unchanged, but its internal diameter will increase, its external diameter will increase, and its thickness will increase. But its density will decrease because the volume increases.
Points To Remember
- Matter is anything that takes up space and has mass.
- The three states of matter are solid, liquid, and gas.
- Matter is made of a large number of tiny particles called molecules.
- A molecule is the smallest particle that can exist freely in nature and still keep the properties of the substance.
- In liquids, the molecules are not tightly packed; the spaces between them are more than in solids, so the liquid molecules can move more freely.
- In gases, the molecules are not tightly packed; the spaces between them are much more than in solids and liquids, so gas molecules can move anywhere in the space.
- In evaporation, a liquid changes into vapor or gas at all temperatures.
- In boiling, a liquid changes into vapor at a fixed temperature called the boiling point.
- When a liquid or gas is heated, it expands because the molecules gain energy from heat and move apart.
- A solid expands when heated; this is called thermal expansion.
- A solid expands in length, area, and volume when heated; this depends on its original length, area, or volume, the rise in temperature, and the material of the solid.
- The increase in length of a solid is Lₜ - L₀ = L₀ α t, where α is the coefficient of linear expansion.
- The increase in area of a solid is Aₜ - A₀ = A₀ β t, where β is the coefficient of superficial expansion.
- The increase in volume of a solid is Vₜ - V₀ = V₀ γ t, where γ is the coefficient of cubical expansion.
- The coefficients of linear, superficial, and cubical expansion are related as α : β : γ = 1 : 2 : 3.
- When molecules in a solid start vibrating about their mean positions on heating, the solid expands because the molecules move farther apart.
- Some solids, like pyrex glass and quartz, have very little expansion when heated.
- Liquids expand more than solids when heated.
- Liquids only have cubical expansion because they do not have a fixed shape.
- The increase in volume of a liquid is Vₜ - V₀ = V₀ γ t, where γ is the coefficient of cubical expansion.
- Different liquids expand by different amounts when heated to the same rise in temperature; for example, alcohol expands more than water.
- Water contracts on heating from 0°C to 4°C and then expands when heated above 4°C.
- Thermal expansion of liquids is used in devices like a mercury thermometer.
- Gases expand much more than solids and liquids when heated; they also have only cubical expansion.
- On heating, the density of solids, liquids, and gases decreases because their volume increases.
- Water has its maximum density at 4°C, which is 1000 kg/m³.
|
89 docs|6 tests
|
FAQs on Heat Transfer Chapter Notes - Food & Nutrition for Year 6
| $1. What is the effect of heat on molecular motion? |  |
| $2. What is the difference between evaporation and boiling? |  |
| $3. How does temperature affect the thermal expansion of matter? |  |
| $4. How does thermal expansion differ in solids, liquids, and gases? |  |
| $5. What is expansivity, and how does it compare in solids, liquids, and gases? |  |





















