Electrostatics Chapter Notes | Technical Science for Grade 10 PDF Download
| Table of contents |

|
| Two Kinds of Charge |

|
| Law of Attraction and Repulsion |

|
| Van de Graaff Generator |

|
| Charge Conservation |

|
| Points to Remember |

|
| Difficult Words |

|
| Summary |

|
Two Kinds of Charge
This section explores the concept of static electricity, the behavior of positive and negative charges, and how charges are generated and detected, using historical context and tools like the gold-leaf electroscope and van de Graaff generator.
Static Electricity
Definition: Static electricity is the buildup of electric charge on the surface of objects, often caused by rubbing two materials together.Examples:
- Hearing crackling sounds or seeing sparks when removing nylon clothing in the dark.
- Feeling a shock when touching a metal doorknob after walking on a carpet.
- Hair standing up after combing due to charge buildup.
- Dust sticking to plastic objects like lunch boxes or TV screens.
Cause: These phenomena occur when electrons are transferred between materials, creating a separation of positive and negative charges.
Historical Context of Electricity
Origin of the Word: The term "electricity" comes from the Greek word "elektron," meaning amber, a substance known to attract small objects like paper or hair when rubbed with cloth or fur.Early Observations: Thousands of years ago, people noticed static electricity but could not explain it, often confusing it with magnetism.
18th Century Advances: In the 1700s, scientists in Europe and America built machines to produce sparks and experimented with static electricity.
Benjamin Franklin: Invented the lightning rod to protect buildings from lightning strikes, a practical application of static electricity control.
Challenges: Even brilliant scientists struggled to understand what electricity was until the discovery of atoms and electrons.
Electrons and Charges
Atomic Structure Recap (from Chapter 9):- All materials are made of atoms, which consist of a positively charged nucleus (containing protons) and negatively charged electrons orbiting the nucleus.
- An atom is tiny (a million could fit across a human hair) and is electrically neutral when the number of protons equals the number of electrons.
Charge Generation:
- When two materials (e.g., a plastic straw and dry skin) are rubbed together, electrons can transfer from one material to the other.
- The material gaining electrons becomes negatively charged (surplus of electrons).
- The material losing electrons becomes positively charged (deficiency of electrons).
Example: Rubbing a plastic straw with dry skin transfers electrons to the straw (negative charge) and leaves the skin with fewer electrons (positive charge).
Other Materials: Perspex and silky cloth, polythene and wool, or rubber and fur also generate charges when rubbed together.
Law of Attraction and Repulsion
Principle:- Like Charges Repel: Two negative charges or two positive charges push each other away.
- Unlike Charges Attract: A positive charge and a negative charge pull toward each other.
Electric Force: The force between charges is called the electric force, which acts without physical contact (a non-contact force).
Explanation:
- If two plastic straws are rubbed with tissue paper, both gain electrons (negative charge) and repel each other.
- The tissue paper, losing electrons, becomes positively charged and attracts the negatively charged straws.
Nett Charge:
- An object with more electrons than protons has a nett negative charge.
- An object with fewer electrons than protons has a nett positive charge.
- Example: If an object has 10 million positive charges and 9 million negative charges, its nett charge is +1 million.
Gold-Leaf Electroscope
Description: A gold-leaf electroscope is a device used to detect the presence and type (positive or negative) of electric charge.Structure:
- A metal plate at the top, connected to a conducting stem.
- Two thin gold leaves attached to the bottom of the stem, housed in a glass container to protect from air currents.
- A plastic stopper insulates the stem.
How It Works:
- When uncharged, the gold leaves hang down.
- Positive Charge Detection: Bringing a positively charged Perspex rod near the plate attracts electrons from the leaves to the plate, leaving the leaves with a nett positive charge. The leaves repel each other and diverge.
- Negative Charge Detection: Bringing a negatively charged polythene rod near the plate repels electrons from the plate to the leaves, giving the leaves a nett negative charge. The leaves repel each other and diverge.
Identifying Charges:
If the electroscope is positively charged (e.g., from a Perspex rod), bringing an unknown charged object near the plate can identify its charge:
- If the leaves diverge further, the object is positively charged (more electrons are attracted to the plate).
- If the leaves converge, the object is negatively charged (electrons are repelled to the leaves).
Van de Graaff Generator
Description: A van de Graaff generator is a machine that separates large quantities of positive and negative charges, storing them on a metal dome.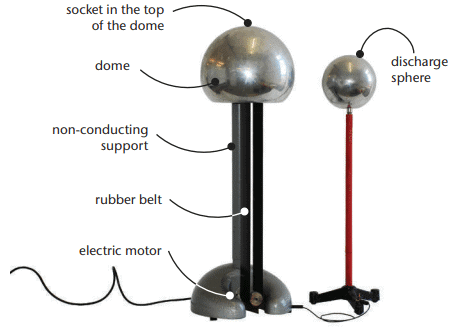 Structure:
Structure:
- A large metal sphere (dome) collects charges.
- A rubber belt moves around a plastic roller, driven by a pulley (manually or by motor).
- Thin wires near the belt collect electrons, which spread across the dome, giving it a negative charge.
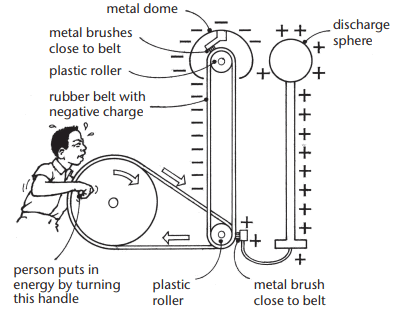 The inside of a van de Graaff generator
The inside of a van de Graaff generator
Operation:
- As the belt moves, electrons accumulate on the dome, creating a high voltage (e.g., 150,000 volts).
- When the charge is large enough, sparks jump from the dome to an earthed object, as air becomes conductive.
Observations:
- Paper strips on the dome repel each other due to like charges.
- Soap bubbles near the dome gain the same charge and repel the dome.
- A person touching the dome has their hair stand out due to like charges repelling each other.
Safety: The high voltage produces a small current, so it does not harm humans.
Lightning Rod Effect: A sharp-pointed rod on the dome prevents charge buildup, as charges leak off the point into the air, similar to how lightning rods on buildings prevent lightning strikes by dissipating charges.
Charge Conservation
This section explains the principle of charge conservation, how charges are measured, and how capacitors store energy by separating charges, including the historical discovery of the Leyden jar.
Measuring Electric Charge
Unit of Charge: The coulomb (C) is the unit for measuring electric charge.- One coulomb equals 6.25 × 1018 (6.25 billion billion) positive or negative charges.
- Symbol: Charge is denoted by Q or q, measured in coulombs (C).
Analogy: Just as sugar is measured in teaspoons rather than grains, charge is measured in coulombs rather than individual electrons or protons.
Context: Every object has countless positive (protons) and negative (electrons) charges due to its atoms, but we measure the nett charge in coulombs.
Principle of Charge Conservation
Definition: The principle of charge conservation states that the nett charge in an isolated system remains constant during any physical process.Isolated System: A system where no charges enter or leave, such as a person and a straw on a non-conducting surface.
Explanation:
- In an isolated system, the total number of positive and negative charges stays the same, even if charges are transferred between objects.
- For every positive charge created, a negative charge is also created, maintaining a nett charge of zero.
Example:
- When rubbing a straw with fingers, the straw gains negative charges (electrons), and the fingers gain positive charges (electron deficiencies). The system’s nett charge remains zero.
- In a gold-leaf electroscope, a charged rod separates positive and negative charges, but the electroscope’s total charge remains zero.
Charge Sharing Between Conductors
Scenario: When two identical conducting spheres with different charges touch, they share their nett charge equally.Mechanism:
- Charges (electrons or electron deficiencies) move between the spheres until the charge is evenly distributed.
- Example: If Sphere 1 has one extra negative charge and Sphere 2 has one extra positive charge, when they touch, the excess electron from Sphere 1 moves to Sphere 2, neutralizing both spheres (net charge zero each).
Formula: For two identical conductors, the final charge on each after touching is:
Qfinal = (Q1 + Q2) / 2
- Q1 and Q2 are the initial charges on the spheres.
- This applies only to identical conductors (same size and shape), as charges do not move across non-conductors.
Worked Examples:
- Two spheres with +3 C and +1 C:
Qfinal = (3 + 1) / 2 = 2 C each. - Two spheres with +3 C and -1 C:
Qfinal = (3 + (-1)) / 2 = 1 C each.
Limitation: The formula does not apply to non-conductors (e.g., charged golf balls), as charges cannot move freely.
Capacitors and Charge Storage
Definition: A capacitor is an electrical device that stores energy by separating positive and negative charges on two conducting plates.Structure:
- Two conducting plates separated by a non-conducting material (dielectric, e.g., plastic or air).
- Example: In a Leyden jar, one plate is foil inside a bottle, and the other is foil outside, with plastic or glass as the dielectric.
How It Works:
- A battery or charge generator pushes electrons onto one plate (negative charge) and removes electrons from the other (positive charge).
- The plates hold equal amounts of opposite charges due to charge conservation.
- Electrons repel each other, so the flow stops when the plate is fully charged, storing potential energy due to the attraction between opposite charges.
Applications:
- Absorb energy to prevent sudden high voltages.
- Store energy for starting electric motors.
- Can power a circuit (e.g., light a bulb or LED) like a battery.
Increasing Capacity: Larger plates or a better dielectric allow a capacitor to store more charge.
Manufacturing: Modern capacitors use rolled-up foil sheets with a dielectric to maximize plate area in a small space.
Discovery of the Leyden Jar
Historical Context: In 1746, Pieter von Muschenbroek in Leyden, Holland, discovered the first capacitor, called a Leyden jar.Experiment:
- A glass sphere was spun to generate charge, transferring electrons via a brass chain and iron bar to water in a flask.
- Assistant Andreas Cuneus touched a wire in the flask and received a powerful shock, demonstrating stored charge.
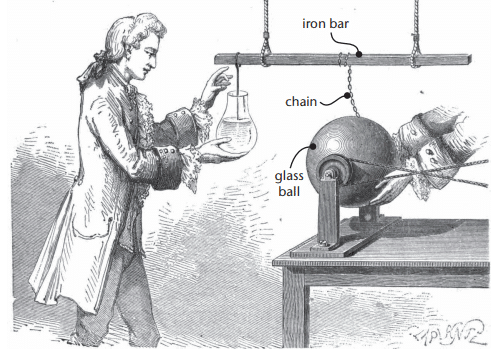
Assistant Andreas is going to touch that wire!
Impact: The Leyden jar allowed scientists worldwide to store and study electric charge, advancing electrostatics research.
Modern Version: Uses aluminium foil inside and outside a plastic bottle, with a round-head fastener to collect charge, replacing water for better conductivity.
Points to Remember
- Static electricity occurs when electrons transfer between materials, creating positive (electron deficiency) and negative (electron surplus) charges.
- The word "electricity" comes from the Greek "elektron" (amber), which attracts objects when rubbed.
- Atoms are neutral when protons equal electrons; rubbing materials can make one positively charged and the other negatively charged.
- Like charges (positive-positive or negative-negative) repel, while unlike charges (positive-negative) attract, via the electric force.
- The gold-leaf electroscope detects charge type: leaves diverge when positive or negative charges are applied, and further divergence indicates the same charge.
- The van de Graaff generator stores large charges on a dome, producing sparks or repelling objects with like charges.
- Lightning rods with sharp points prevent charge buildup by leaking charges into the air, reducing lightning strikes.
- The coulomb (C) measures charge, with 1 C equaling 6.25 × 1018 charges.
- The principle of charge conservation states that the nett charge in an isolated system remains constant.
- When two identical conductors touch, they share charge equally: Qfinal = (Q1 + Q2) / 2.
- A capacitor stores energy by separating equal amounts of positive and negative charges on two plates, used in circuits to manage voltage or power devices.
- The Leyden jar, discovered in 1746, was the first capacitor, enabling early electrostatic experiments.
Difficult Words
- Static Electricity: The buildup of electric charge on an object’s surface, often from rubbing materials together.
- Electron: A negatively charged particle orbiting an atom’s nucleus.
- Proton: A positively charged particle in an atom’s nucleus.
- Nett Charge: The overall charge of an object after adding positive and negative charges (e.g., more electrons than protons = negative nett charge).
- Electric Force: The non-contact force causing like charges to repel and unlike charges to attract.
- Gold-Leaf Electroscope: A device with a metal plate and gold leaves to detect and identify electric charges.
- Van de Graaff Generator: A machine that separates and stores large electric charges on a metal dome, producing high voltages.
- Coulomb (C): The unit of electric charge, equal to 6.25 × 1018 positive or negative charges.
- Charge Conservation: The principle that the nett charge in an isolated system remains constant.
- Isolated System: A system where no charges enter or leave, maintaining constant nett charge.
- Capacitor: A device with two conducting plates that stores energy by separating positive and negative charges.
- Dielectric: A non-conducting material between a capacitor’s plates, enabling charge storage.
- Leyden Jar: The first capacitor, discovered in 1746, used to store electric charge for experiments.
Summary
Chapter 12 explores electrostatics, focusing on how electric charges behave and are conserved. Static electricity arises when rubbing materials transfers electrons, creating positive (electron-deficient) and negative (electron-surplus) charges. Like charges repel, and unlike charges attract, via the electric force, as seen when charged straws or paper repel or attract. The gold-leaf electroscope detects charge types by the divergence of its leaves, while the van de Graaff generator stores large charges, demonstrating repulsion and sparking. Lightning rods prevent strikes by leaking charges from sharp points. The coulomb measures charge, and the principle of charge conservation ensures that the nett charge in an isolated system remains constant. When identical conductors touch, they share charge equally, calculable by Qfinal = (Q1 + Q2) / 2. Capacitors, like the historical Leyden jar, store energy by separating opposite charges on plates, used in electrical systems to manage energy. These concepts explain how charges interact and are harnessed in technology.
FAQs on Electrostatics Chapter Notes - Technical Science for Grade 10
| 1. What are the two kinds of electric charge? |  |
| 2. How does the law of attraction and repulsion work in electrostatics? |  |
| 3. What is the function of a Van de Graaff generator? |  |
| 4. What is charge conservation in electrostatics? |  |
| 5. What are some difficult words related to electrostatics that students might need to understand? |  |



















