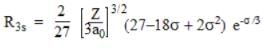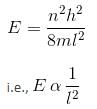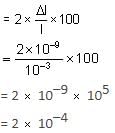GATE Life Science Mock Test- 1 (Botany & Zoology) - GATE Life Sciences MCQ
30 Questions MCQ Test GATE Life Sciences 2026 Mock Test Series - GATE Life Science Mock Test- 1 (Botany & Zoology)
According to research, listening to music while exercising enhances performance and alleviates discomfort. Scientists investigated if listening to music during study sessions could improve students' learning outcomes, but the findings were inconclusive. It was observed that students who required external stimulation for studying performed worse, whereas those who did not require any external stimulation experienced benefits from music.
Which of the following statements is the CORRECT inference from the passage above?
The expression for the radial wave function of the hydrogen atom corresponding to the 3s orbital is given by __________.
The proper sequence of Cr2O3, CrO3, MgO, and MnO, arranged by their increasing base strength, is
The major product Q resulting from the following reaction has the structure shown below:
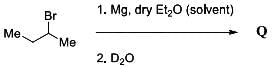
The energy of an electron in its ground state within a hydrogen atom is –13.60 eV. What is the energy of the electron in the third excited state, expressed in eV (rounded to two decimal places)?
A particle is restricted to a one-dimensional box measuring 1 mm in length. If the length is altered by 10–9 m, what is the percentage change in the ground state energy?
The proper order of acidity for the compounds depicted below is
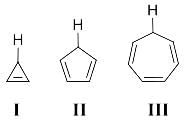
Consider a reaction represented as X → Products.
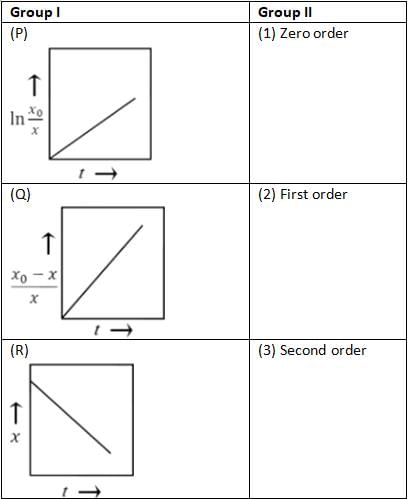
Group I presents three graphs depicting the concentrations of the reactant over time, where x signifies the concentration of reactant X at time t, and x0 denotes the initial concentration of reactant X at time t = 0. Group II lists various reaction orders. Match each graph with the corresponding order of reaction.
Given the following standard heats of formation, ∆fH⦵(P, g) = 314.6 kJ mol−1, ∆fH⦵(PH3, g) = 5.4 kJ mol−1, and ∆fH⦵(H, g) = 218.0 kJ mol−1, the average bond enthalpy of a P–H bond in PH3(g) is __________ kJ mol−1 (rounded off to one decimal place).
Considering the standard reduction potentials, 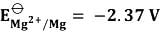 and
and 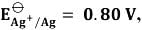 , determine the potential of the following electrochemical cell: Ag (aq., 1 mM) Mg(s) ↔ Ag(s) Mg2 (aq., 0.2 M) at 25°C, which is _______ V (rounded to two decimal places).
, determine the potential of the following electrochemical cell: Ag (aq., 1 mM) Mg(s) ↔ Ag(s) Mg2 (aq., 0.2 M) at 25°C, which is _______ V (rounded to two decimal places).
(Given: Faraday constant = 96500 C mol−1, Gas constant R = 8.314 JK−1mol−1)
The freezing point of 80 g of acetic acid (freezing point constant 3.9 K kg mol−1) was depressed by 7.8 K upon adding 20 g of a certain compound. The molar mass of this compound is _____________ g mol−1 (rounded to the nearest integer).
In Mendel's experiments, the dwarf pea mutant exhibited a deficiency in which of the following enzymes that play a role in the synthesis of gibberellins?
Which of the following enzyme(s), when expressed in excess, would lead to an increase in the β-carotene content of rice grains?
The dihybrid phenotypic ratio of 9 : 3 : 4 is associated with which type of genetic interactions?
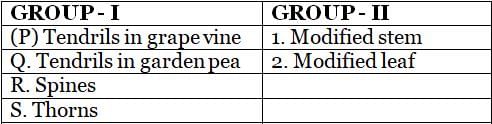
Pair the altered organs in GROUP I with their respective prototypical forms in GROUP II and select the CORRECT option.
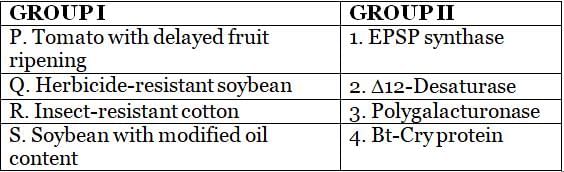
Associate each genetically modified crop from GROUP I with its relevant genetic element in GROUP II.
Which of the following option(s) is/are CORRECT regarding the generation of hybrid plants utilizing male sterile lines based on Barnase/Barstar technology?
In a diploid species of plants, the T allele results in tall individuals and is entirely dominant over the t allele, which leads to short individuals. Likewise, the W allele causes the formation of round seeds and is fully dominant over the w allele that produces wrinkled seeds (assuming the T and W loci are not linked). If a plant with a TTWW genotype is crossed with another plant having a ttww genotype, what fraction of the F2 generation generated from the combination of both recombinant gametes would be ___________? (Round your answer to two decimal places.)
During which period did Archaeopteryx face extinction?
Infectivity in microfilariae occurs within
A man, whose parents have blood groups A and O respectively, marries a woman with blood group AB. If the man possesses blood group A, then the total number of distinct blood groups that their children could inherit will be _________ (in integer).
In a polypeptide's structure, a single α-helix (3.613 helix) consists of 32 intrachain hydrogen bonds. The total number of turns present in the helix will be _________ (in integer).
In a population of snakes residing on a remote island, the gene under consideration is in Hardy–Weinberg equilibrium and consists of two alleles (A and a). Given that the frequency of allele A is 0.6, what is the frequency of genotype Aa? Please round your answer to two decimal places.
The high frequency of the allele linked to sickle-cell anemia in certain human populations is likely attributed to
The process of ubiquitination of mitotic cyclins is carried out by which of the following?
CheA, a protein involved in bacterial chemotaxis
P. acts as a histidine kinase
Q. exhibits autophosphorylation
R. transfers phosphoryl groups to conserved aspartate residues in CheY
S. functions as a tyrosine kinase
Which glycolytic enzyme is inhibited by iodoacetate?
Which of the following options identifies the animals that are endemic to India?
In a testcross experiment, the distances between locations A and B are 10, and between B and C are 20. If a double crossover occurs at a rate of 1.6%, determine the coefficient of coincidence.
(Calculate your answer to one decimal place.)
An enzyme facilitates the transformation of 30 µM of a substrate into product at a reaction velocity of 9.0 µM s-1. Given that [Et] = 30 nM and Km = 10 µM, the ratio Kcat / Km for the enzyme will be n × 107 M-1s-1. What is the value of n in integer form?