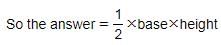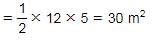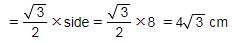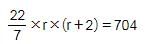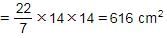DSE Odisha TGT Science PCM Mock Test - 8 - OTET MCQ
30 Questions MCQ Test DSE Odisha TGT Mock Test Series 2025 - DSE Odisha TGT Science PCM Mock Test - 8
Grid Corporation of Odisha (GRIDCO) was incorporated on
Which branch of the Indian Armed Forces is primarily responsible for securing Indian airspace?
The basin of Talcher coalfield mainly occupies the
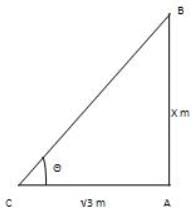
At a moment, the shadow's length of a shaft is √3 times the stature of the shaft. The edge of rise of the sun is:
What is the area of a right-angled triangle with 12 meter base and 13 meter hypotenuse?
What is the height of an equilateral triangle with 8 cm side?
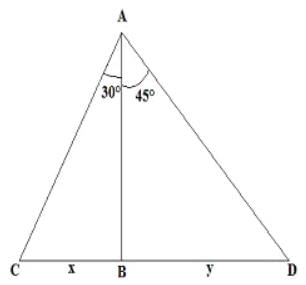
Two boats are spotted on the two sides of a light house. If the angle of depression made by both the boats from top of the lighthouse is 30° and 45° and the height of the light house is 125 m then find the distance between the two boats.
The area and the perimeter of a circle are x cm2 and y cm respectively. If ‘x + y = 704’, what is the area of the circle?
What is the shortcut key to insert current date in a cell?
What is the shortcut key to hide entire column?
Directions: Answer the following question by selecting the most appropriate option.
Learning disabilities may occur due to all of the following except
Which of the following is not an agent of socialisation process in the life of a learner?
'Education-of-all-in-schools-for-all' could be a tagline for which of the following?
What are the most important effects on the development of the child's brain?
In the process of teaching, a teacher should always try to:
Which of the following forms a virtual and erect image for all positions of the object?
An unpolarised beam of intensity 2a2 passes through a thin polaroid. Assuming zero absorption in the polaroid, the intensity of emergent plane polarised light will be
The bluish color predominates in a clear sky,
An cation A3+ has 18 electrons. Write the atomic number of A.
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1.
An orbital is described with the help of a wave function. Since many wave functions are possible for an electron, there are many atomic orbitals. When atom is placed in a magnetic field the possible number of orientations for an orbital of azimuthal quantum number 3 is:
Two values of spin quantum numbers i.e., +1/2 and -1/2 represent