AWES PGT Chemistry Mock Test - 10 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2025 - AWES PGT Chemistry Mock Test - 10
Which country is poised to assume the role of the chairman of the International Sugar Organisation (ISO) in 2024?
Who won the gold medal in the women's singles event at the 2023 World Badminton Championships?
Which of the following is the first biosphere reserve of India?
Difficulty in recalling sequence of letters in words and frequent loss of visual memory is associated with_____.
Select the appropriate techniques favourable for motivating students for learning in the classroom.
(i) Encouraging maximum participation of students in the classroom
(ii) Encourage competition for marks
(iii) Help students set goals based on their interest
(iv) Giving freedom to students for expressing their ideas
Choose the correct option.
The lowest region of atmosphere in which the human being along with other organisms live is known as:
When diazonium salt solution is treated with water at a temperature of 283 K it forms?
What is the major final product of the following sequence of reaction?
The conversion of primary aromatic amines into diazonium salts is known as:
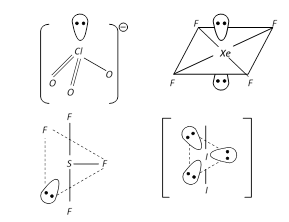
Among the following compounds the maximum number of lone pair is present on the central atom of:
amongst the following compounds, the optically active alkane having lowest molar mass is
[AIEEE 2004]
Out of Cr (VI) as
, which is better oxidising agent?
ΔCH° (cyclopropane) = - 2091.97 kJ mol-1
ΔCH° (propene) = - 2058.32 kJ mol-1
Q. What is ΔrH° (in kcal) propene → cyclopropane?
Oxirane is a reactive ether, undergo nucleophilic attack at a-carbon in the presence of both acidic and basic medium. The generalised mechanism in the two medium are :
I. Acidic medium
II. Basic medium
As shown above, in acidic medium, nucleophilic attack occur at more substituted α-carbon while in basic medium, nucleophilic attack occur preferably at less substituted α-carbon.
Q.
If X is heated with concentrated H2SO4 a cyclic compound is formed which is
A 20.0 litre vessel initially contains 0.50 mole each of H2 and I2 gases. These substances react and finally reach an equilibrium condition. Calculate the equilibrium concentration of HI if Keq = 49 for the reaction H2 + I2 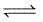 2HI.
2HI.
Which type of a property is the Brownian movement of colloidal solution?
Assuming (2s-2p) mixing is not operative, the paramagnetic species among the following is
[JEE Advanced 2014]
We have
I. 25 mL of 1 M NaOH
II. 10 mL of 0.50 M NaCI
On mixing the two solutions, molar concentrations of Na+, OH- and Cl- respectively, are
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:
An element with atomic number will form a basic oxide________
Increasing order of oxidation number of oxygen is found in
What is the acute angle between oxygen and hydrogen in the solid form of hydrogen peroxide?
|
12 docs|30 tests
|