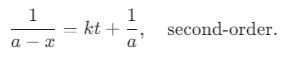VITEEE PCBE Mock Test - 4 - JEE MCQ
30 Questions MCQ Test VITEEE: Subject Wise and Full Length MOCK Tests - VITEEE PCBE Mock Test - 4
What distinguishes liverworts from mosses in particular?
Nervous System and Sense Organs Which statement correctly describes the nervous system or sense organs of frogs?
Given below are two statements : One is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : A flower is defined as modified shoot wherein the shoot apical meristem changes to floral meristem.
Reason R : Internode of the shoot gets condensed to produce different floral appendages laterally at successive node instead of leaves.
In the light of the above statements, choose the correct answer from the options given below :
Identify the step in tricarboxylic acid cycle, which does not involve oxidation of substrate?
What would be the cardiac output of a person having 72 heart beats per minute and a stroke volume of 50 ml?
A typical lower with superior ovary and other floral parts inferior is called:
Which hormone is released by the JG cells in response to a fall in glomerular blood flow?
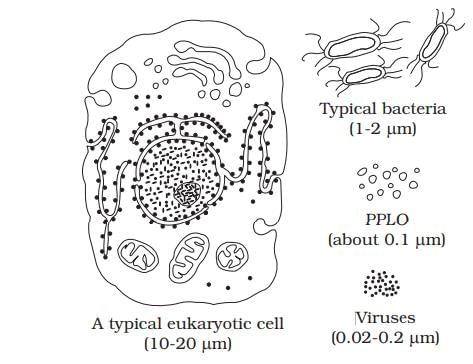
Arrange the following in the increasing order of their size:
If the charge on an electron is 1.6 x 10-19 C, then the number of electrons passing through a section of wire per second, when the wire carries a current of 1/4 ampere, is
When a charged particle enters a uniform magnetic field at a right angle to the direction of the field, which of the following change(s)?
Earth is assumed to be a sphere of radius R. A platform is arranged at a height R from the surface of Earth. The escape velocity of a body from this platform is fve, where ve is its escape velocity from the surface of Earth. The value of f is
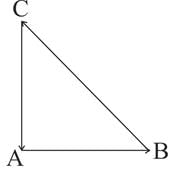
Three forces start acting simultaneously on a particle moving with velocity 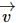 . These forces are represented in magnitude and direction by the three sides of triangle ABC (as shown). The particle will now move with velocity
. These forces are represented in magnitude and direction by the three sides of triangle ABC (as shown). The particle will now move with velocity
Two periodic waves of intensities I₁ and I₂ pass through a region at the same time in the same direction. The sum of the maximum and minimum intensities is:
A square loop is made by a uniform conductor wire as shown in figure
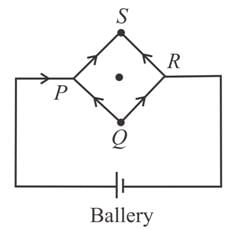
The net magnetic field at the centre of the loop if side length of the square is a
Consider the following half reactions:
Zn2+ + 2e- → Zn(s); E0 = -0.76V
Cu2+ + 2e- → Cu(s); E0 = -0.34V
Which of the following reactions is spontaneous?
Initially, only A is present, and its concentration is A0. Assume At and Aeq are the concentrations of A at time 't' and at equilibrium, respectively. The time 't' at which At = (A0 + Aeq)/2 is
The hybridisation of the central atom and the shape of [IO2F5]2 ion, respectively, are
Consider : a = initial concentration
a - x = current concentration at a time t
A straight line is drawn taking 1/a-x on y-axis and time on x-axis with a slope equal to rate constant with an intercept 1/a on y-axis. What is the order of the reaction?
The chemical reaction, 2AgCI(g) + H2(g) → 2HCI(aq) + 2Ag(s) taking place in a galvanic cell is represented by the notation:
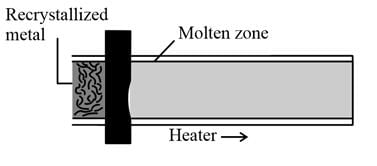
The method of zone refining of metals is based on the principle of
Among the following isomeric C4H11N amines, one having the lowest boiling point
Transition metals do not show the highest oxidation state with fluorine, but they do so with oxygen. What would be the proper reason for the same?
Which of following is true about equity ownership in different countries?
Directions: Choose the word/group of words which is most similar in meaning to the word printed in underline as used in the passage.
Concentrated
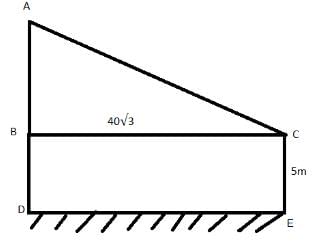
From the top of a platform 5 metre high, the angle of elevation of a tower is 30 degrees. If the platform is positioned 40√3 metres away from the tower, how tall is the tower?
|
1 videos|7 docs|63 tests
|
|
1 videos|7 docs|63 tests
|




 =
= 

 = 0.625 x 1019 x 1/4
= 0.625 x 1019 x 1/4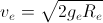
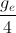 .
.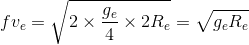
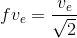

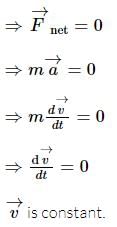
 remains unchanged.
remains unchanged.

 and xeq. = A0 - Aeq
and xeq. = A0 - Aeq