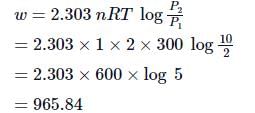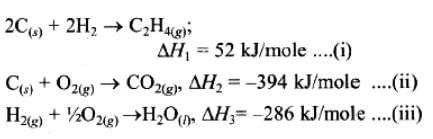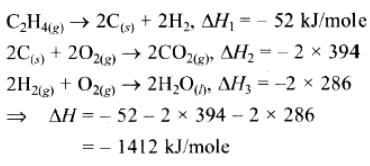Test: Thermodynamics And Thermochemistry - 1 - Chemistry MCQ
20 Questions MCQ Test Physical Chemistry - Test: Thermodynamics And Thermochemistry - 1
Heat produced in calories by the combustion of one gram of carbon is called:
The temperature of the system decreases in an:
For the isothermal expansion of an ideal gas:
In an isochoric process, the increased internal energy is:
The process in which no heat enters or leaves the system is termed as:
If in a container neither mass and nor heat exchange occurs then it constitutes a ______.
Which of the following is true for an adiabatic process:
Among the following, intensive property is:
For the reaction of one mole of zinc dust with one mole of sulphuric acid in a bomb calorimeter, ΔU and W correspond to:
Which of the following expressions represent the first law of thermodynamics:
At 270C one mole of an ideal gas is compressed isothermally and reversibly from a pressure of 2 atm to 10 atm. The value of ΔE and q are (R = 2):
The heat required to raise the temperature of a body by 1K is called:
Which of the following is true for the reaction H2O(l) ⇋ H2O(g) at 1000C at one atmosphere:
Identify the correct statement regarding entropy:
Maximum entropy will be in which of the following:
If enthalpies of formation C2H4 (g), CO2 (g) and H2O (l) at 250C and 1 atm. pressure be 52, –394 and –286 KJ mol–1 respectively. The enthalpy of combustion of C2H4 (g) will be:
Heat of neutralization of strong acid and weak base is:
The heat evolved in the combustion of methane is given by the following equations:
CH4 (g) + 2O2 (g) → CO2 (g) + H2O (l) ΔH = -890.3 KJ
How many grams of methane would be required to produce 444.15 KJ of heat of combustion:
One gram sample of NH4NO3 is decomposed in a bomb calorimeter. The temperature of the calorimeter increases by 6.12 K. The heat capacity of the system is 1.23 kJ/g/deg. What is the molar heat of decomposition for NH4NO3?
|
90 videos|144 docs|67 tests
|