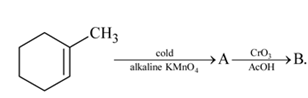Test: Reactions of Alcohols - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Reactions of Alcohols
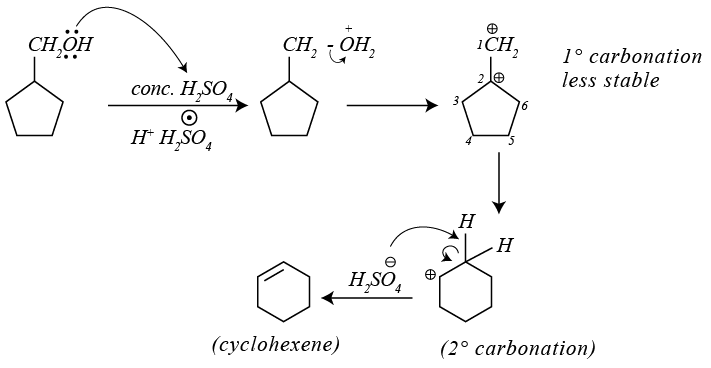
Dehydration of alcohols by conc. H2SO4 takes place according to following steps:
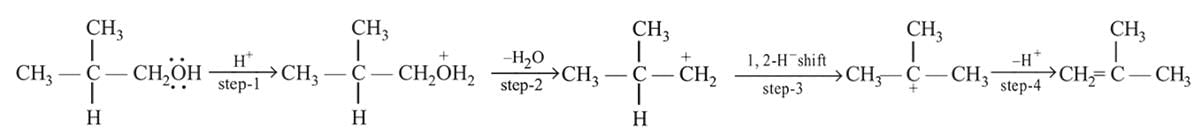
The slowest and fastest steps in the above reaction are

The slowest and fastest steps in the above reaction are
The most probable product in the reacion given below is

Which of the following alcohols is dehydrated most readily with conc. H2SO4.
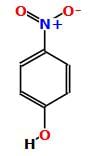
Which one of the following compounds has the most acidic nature?
Arrange the following in increasing order of their acidity?
(a) o -cresol, (b) salicylic acid, (c) phenol
An organic compound A reacts with methyl magnesium iodide to form an addition product which on hydrolysis forms the compound B. Compound B gives blue colour salt in Victor Meyer's test. The compounds A and A are respectively
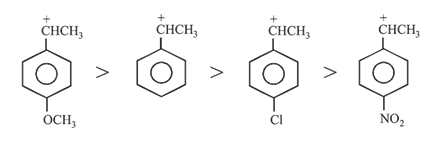
The strongest acid among the following aromatic compounds is
Which compound is formed when CH3OH reacts with CH3 - Mg - X
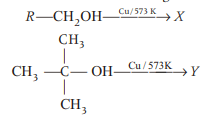
Methyl alcohol can be distinguished from ethyl alcohol using
The reagent which easily reacts with ethanol and propanol is
CH₃CH₂OH → CH₃CH₂Cl
Find out the number of reagents that can be used for the above transformation from the following:
HCl/ZnCl₂, P + Cl₂/Δ, SOCl₂, TsCl, NaCl, PCl₃, PCl₅, AgCl
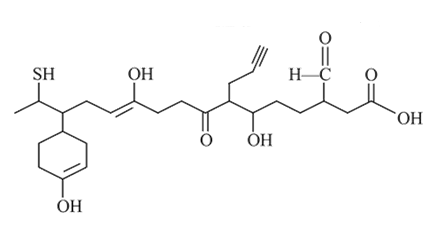
Find number of moles of CH4 obtained when 1 mole of the given compound reacts with excess of CH3MgBr
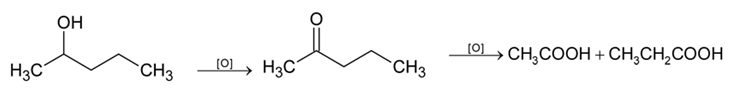
An alcohol on oxidation is found to give CH₃COOH and CH₃CH₂COOH. The structure of the alcohol is:
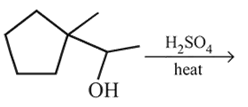
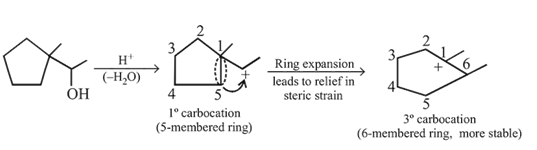
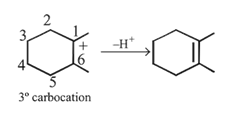
Dehydration of cyclopentyl carbocation with conc. H2SO4 forms
|
361 videos|822 docs|301 tests
|


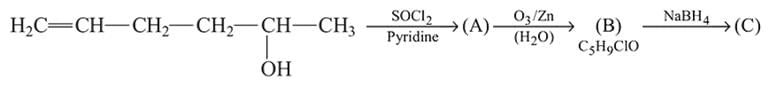
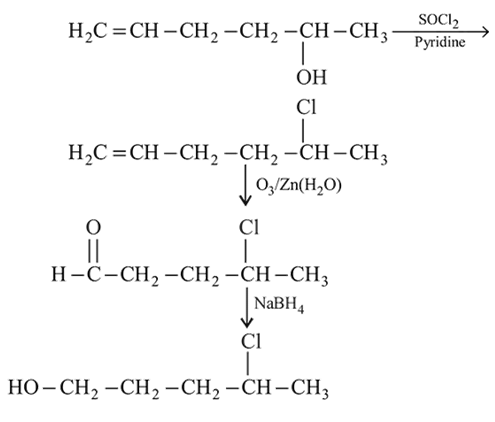

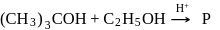
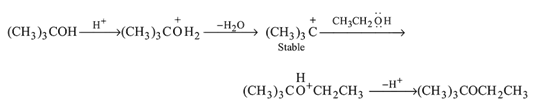
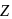 in the following sequence of reactions?
in the following sequence of reactions?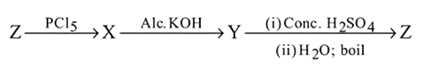
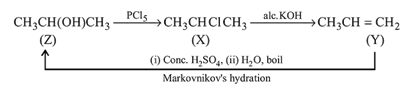
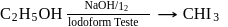 (yellow ppt)
(yellow ppt)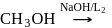 No ppt.
No ppt.