AWES PGT Chemistry Mock Test - 5 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2025 - AWES PGT Chemistry Mock Test - 5
Recently, India surpassed which country to become the fourth-largest equity market globally?
_____ motives deal with the need to reach satisfying feeling states and to obtain personal goals.
What happens to atomic radius on going from left to right in a period in a periodic table?
Consider the following aldol condensation reaction,
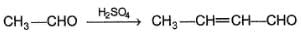
Q.
The nucleophile is
Based on the following thermochemical reactions at 298 K and 1 bar
Q. Enthalpy of vaporisation of H2O (l) is
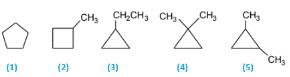
How many total cyclic isomers are possible for C5H10 ?
An ion M2+, forms the complexes [M(H2O)6]2+, [M(en)3]2+ and [MBr6]4-, match the complex with the appropriate colour.
1 M NaCl and 1 M HCl are present in an aqueous solution. The solution is
A metal in a compound can be displaced by another metal in the uncombined state. Which metal is a better reducing agent in such a case?
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q.Oxidation number of the Fe in the ring is
At 675 K, H2(g) and CO2(g) react to form CO(g) and H2O(g), Kp for the reaction is 0.16. If a mixture of 0.25 mole of H2(g) and 0.25 mol of CO2 is heated at 675 K, mole% of CO(g) in equilibrium mixture is :
Heat of hydrogenation of ethene is x1 and that of benzene is x2. The resonance energy of benzene is
The quantum number which specifies the location of an electron as well as energy is
Based on the following thermodynamic data,
Q. On the total mass basis of reactants, which reaction will generate the greatest amount of heat?
The unit of rate constant for a first order reaction is
Arrange the following in decreasing order of their boiling points
A) n-butane
B) 2-methylbutane
C) n-pentane
D) 2,2-dimethylpropane
What is the major organic product in the following reaction?
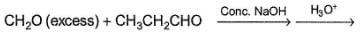
Lanthanide for which + II and + III oxidation states are common is
In the conversion of Br2 to BrO3-, the oxidation number of Br changes from
H2S (g) initially at a pressure of 10.0 atm and a temperature of 800 K, dissociates as
2H2S(g) ⇌ 2H2(g) + S2(g)
At equilibrium, the partial pressure of S2 vapour is 0.020 atm . Thus, Kp is
The chemical reaction in which reactants require high amount of activation energy are generally
Hot conc. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and nonmetals. Which of the following element is oxidised by conc. H2SO4 into two gaseous products?
|
12 docs|30 tests
|



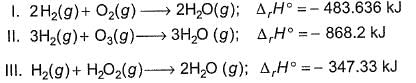 From I, total mass of reactant, 36 gm
From I, total mass of reactant, 36 gm
















