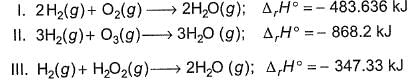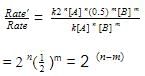AWES PGT Chemistry Mock Test - 4 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2025 - AWES PGT Chemistry Mock Test - 4
With whom has the Government of India signed a loan agreement to strengthen the Fintech eco system in India?
Recently, how many sites have been added to the Global Geoparks network?
When members of the same family become rulers one after the other, the family is called
Which company's rocket was used to launch the TSAT-1A satellite?
In a classroom with diverse group of students, a teacher should ______
‘Choice of challenge’ is a characteristic of which of the following?
Solubility of s block halides in water depends upon:
The reaction of primary amine with Chloroform and ethanoic solution of KOH is called:
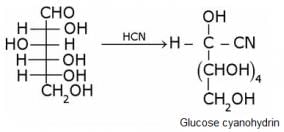
What does the following reaction shows about the structure of glucose?
Identify the correct statements with reference to the given reaction
What is aggregation of colloidal particles into insoluble precipitate by addition of some suitable electrolyte called?
Since the isotopes have the same electronic configuration, they have the same
Based on the following reaction,
It can be concluded that
Which of the following correctly represents H-bonding?
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
Based on the following thermodynamic data,
Q. Which oxidising agent will generate the greatest amount of energy per gram of oxidising agent?
The rate law for a reaction between substances A and B is given by
Rate = k [A]n [B]m
On doubling the concentration of A and halving the concentration of B, the ratio of the new rate to the earlier rate of the reaction will be as–
[AIEEE-2003]

Suitable reagent for following cenversion will be :
Stoichiometric compounds of dihydrogen are formed with
According to the VSEPR theory, the geometry and shape of the molecule depends upon:
10 dm3 of an ideal monoatomic gas at 27° C and 1.01 x 105 Nm-2 pressure are heated at constant pressure to 127°C. Thus entropy change is
The gaseous envelope around the earth is known as atmosphere. The lowest layer of this is extended upto 10 km from sea level, this layer is _________.
Which of the following complex will give white precipitate with barium chloride solution?
Who was the scientist credited with devising the first periodic table similar to the one we use today?
when this equation is balanced, the num ber of OF- ions added
The information that is/are needed to determine the molar mass of an unknown solute is/are
|
12 docs|30 tests
|