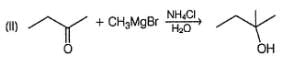AWES PGT Chemistry Mock Test - 1 - AWES TGT/PGT MCQ
30 Questions MCQ Test AWES PGT Mock Test Series 2025 - AWES PGT Chemistry Mock Test - 1
Benisagar, which is in the news recently, is a place located in which state?
Who has become the first Bangladeshi umpire to be appointed to the ICC Elite Panel of Umpires?
Teddy was told by his class teacher that schools are like a social agent and hence, an important part of the society. The class teacher referred to schools as a social agent because
In an experiment conducted on a hungry chimpanzee, some bananas were kept outside its cage, but beyond its reach. Some sticks were also kept in its cage. After several unsuccessful attempts to reach out to the bananas, the chimpanzee pondered over the problem. Then, he picked up a stick and pulled the bananas towards itself. In this case, learning took place by
Which of the following can be termed as mixed complex?
A vessel of 250 litre was filled with 0.01 mole of Sb2S3 and 0.01 mole of H2 to attain the equilibrium at 440°c as
Sb2S3(s) + 3H2(g) 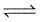 2Sb(s) + 3H2S(g).
2Sb(s) + 3H2S(g).
After equilibrium the H2S formed was analysed by dissolving it in water and treating with excess of Pb2+ to give. 1.195 g of PbS (Molecular weight = 239) precipitate.
What is value of Kc of the reaction at 440°C ?
In which of the following solids, ions of opposite charges are held together by strong electrostatic forces of attraction?
Direction (Q. Nos. 1-15) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the
I. benzene (C6H6) and
II. borazine (B3N3H6)
Select the correct statement.
Among the following enthalpies, which is always less than zero?
Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge of the nucleus (Ze). This is known as
How many gm of solid NaOH must be added to 100 ml of a buffer solution which is 0.1 M each w.r.t. Acid HA and salt Na+ A- to make the pH of solution 5.5. Given pKa(HA) = 5 (Use antilog (0.5)= 3.16)
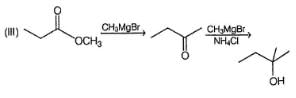
Choose the reagent and reactant that would produce 2-methyl-2-butanol as the major product.
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
During the electrolysis of aqueous Zn(NO3)2 solution
The atom which defines the structure of a family of organic compounds and their properties is called ___________
Substances that are strongly attracted by applied magnetic field and can be permanently magnetized are
Which among the following substances is an example of multimolecular colloids?
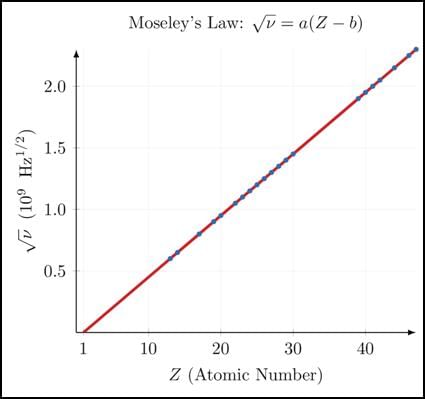
According to Moseley, a straight-line graph is obtained on plotting-
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which is a possible set of quantum numbers for a valence unpaired electrons in ground state atom of phosphorus (Z = 15)?
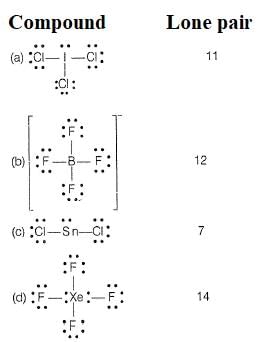
Which of the following molecule/species has the minimum number of lone pairs?
lf En = total energy, Kn = kinetic energy, Vn = potential energy and rn = radius of the nth orbit, then based on Bohr’s theory, match the parameter in Column I with the values in Column II.
Which of the following tests can be used to distinguish between two isomeric ketones: 3- pentanone and 2- pentanone?
Aniline on treatment with sodium hypochlorite gives:
In the earth’s atmosphere, hydrogen exists in the form of
|
12 docs|30 tests
|



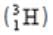 has 1 proton and 2 neutrons.
has 1 proton and 2 neutrons.