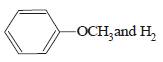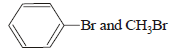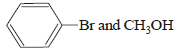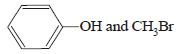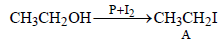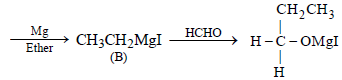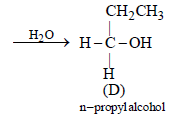All Exams >
JEE >
35 Years Chapter wise Previous Year Solved Papers for JEE >
All Questions
All questions of Alcohols, Phenols and Ethers for JEE Exam
During dehydration of alcohols to alkenes by heating withconc. H2SO4 the initiation step is [2003]- a)formation of carbocation
- b)elimination of water
- c)formation of an ester
- d)protonation of alcohol molecule
Correct answer is option 'D'. Can you explain this answer?
During dehydration of alcohols to alkenes by heating withconc. H2SO4 the initiation step is [2003]
a)
formation of carbocation
b)
elimination of water
c)
formation of an ester
d)
protonation of alcohol molecule
|
|
Devika Choudhury answered |
Dehydration of alcohols to alkenes:
Dehydration of alcohols is a common reaction used to convert alcohols into alkenes. This reaction involves the removal of a molecule of water from the alcohol molecule.
The reaction mechanism:
The reaction proceeds via an acid-catalyzed mechanism, where the alcohol molecule is protonated by a strong acid such as concentrated sulfuric acid (H2SO4) to form an oxonium ion. This oxonium ion is highly unstable and loses a molecule of water to form a carbocation intermediate. The carbocation intermediate is then deprotonated by a base (such as a neighboring water molecule or the conjugate base of the acid catalyst) to form the alkene product.
The initiation step:
In the initiation step of the dehydration reaction, the alcohol molecule is protonated by the acid catalyst (H2SO4). This protonation occurs on the oxygen atom of the alcohol, resulting in the formation of an oxonium ion. This oxonium ion is highly reactive and serves as an intermediate in the reaction.
Why is option 'D' correct?
Option 'D' states that the initiation step of the reaction is the protonation of the alcohol molecule. This is indeed the correct answer. Protonation of the alcohol molecule is the first step in the reaction and leads to the formation of the oxonium ion intermediate.
The protonation of the alcohol molecule occurs because the oxygen atom of the alcohol is a nucleophile and can react with the acid catalyst (H2SO4). The acid catalyst donates a proton to the oxygen atom, resulting in the formation of the oxonium ion. This protonation step is essential for the subsequent loss of water and the formation of the carbocation intermediate.
Conclusion:
In the dehydration of alcohols to alkenes, the initiation step involves the protonation of the alcohol molecule by a strong acid catalyst. This protonation leads to the formation of an oxonium ion, which serves as an intermediate in the reaction. Option 'D' correctly identifies the initiation step of the reaction.
Dehydration of alcohols is a common reaction used to convert alcohols into alkenes. This reaction involves the removal of a molecule of water from the alcohol molecule.
The reaction mechanism:
The reaction proceeds via an acid-catalyzed mechanism, where the alcohol molecule is protonated by a strong acid such as concentrated sulfuric acid (H2SO4) to form an oxonium ion. This oxonium ion is highly unstable and loses a molecule of water to form a carbocation intermediate. The carbocation intermediate is then deprotonated by a base (such as a neighboring water molecule or the conjugate base of the acid catalyst) to form the alkene product.
The initiation step:
In the initiation step of the dehydration reaction, the alcohol molecule is protonated by the acid catalyst (H2SO4). This protonation occurs on the oxygen atom of the alcohol, resulting in the formation of an oxonium ion. This oxonium ion is highly reactive and serves as an intermediate in the reaction.
Why is option 'D' correct?
Option 'D' states that the initiation step of the reaction is the protonation of the alcohol molecule. This is indeed the correct answer. Protonation of the alcohol molecule is the first step in the reaction and leads to the formation of the oxonium ion intermediate.
The protonation of the alcohol molecule occurs because the oxygen atom of the alcohol is a nucleophile and can react with the acid catalyst (H2SO4). The acid catalyst donates a proton to the oxygen atom, resulting in the formation of the oxonium ion. This protonation step is essential for the subsequent loss of water and the formation of the carbocation intermediate.
Conclusion:
In the dehydration of alcohols to alkenes, the initiation step involves the protonation of the alcohol molecule by a strong acid catalyst. This protonation leads to the formation of an oxonium ion, which serves as an intermediate in the reaction. Option 'D' correctly identifies the initiation step of the reaction.
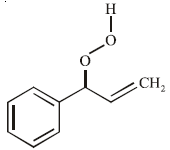
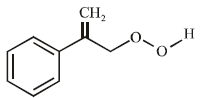
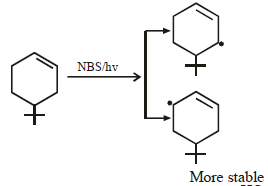
The product of the reaction given below is: [JEE M 2016]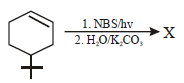
- a)
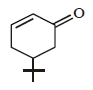
- b)
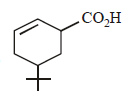
- c)
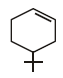
- d)
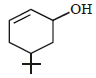
Correct answer is option 'D'. Can you explain this answer?
The product of the reaction given below is: [JEE M 2016]
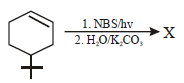
a)

b)
c)

d)


|
Telecom Tuners answered |
N – bromosuccinimide results into bromination at allylic and benzylic positions

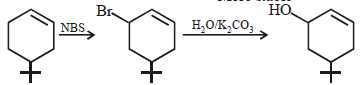
The best reagent to convert pent-3-en-2-ol into pent-3-in-2-one is [2005]- a)Pyridinium chloro-chromate
- b)Chromic anhydride in glacial acetic acid
- c)A acidic dichromate
- d)Acidic permanganate
Correct answer is option 'A'. Can you explain this answer?
The best reagent to convert pent-3-en-2-ol into pent-3-in-2-one is [2005]
a)
Pyridinium chloro-chromate
b)
Chromic anhydride in glacial acetic acid
c)
A acidic dichromate
d)
Acidic permanganate

|
Anand Khanna answered |
Pyridinium chloro chromate (PCC) oxidises an alcohol group selectively in the presence of carbon-carbon double bond.
Arrange the following compounds in order of decreasing acidity :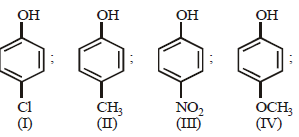
- a)II > IV > I > III
- b)I > II > III > IV
- c)III > I > II > IV
- d)IV > III > I > II
Correct answer is option 'C'. Can you explain this answer?
Arrange the following compounds in order of decreasing acidity :

a)
II > IV > I > III
b)
I > II > III > IV
c)
III > I > II > IV
d)
IV > III > I > II
|
|
Rohit Jain answered |
Electron withdrawing substituents like –NO2, Cl increase the acidity of phenol while electron releasing substituents like – CH3, – OCH3 decreases acidity. hence the correct order of acidity will be
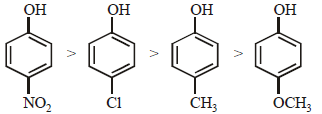
Further (– I) NO2 > (–I) Cl and (+ I) CH3 > (+I) OCH3
The product of acid catalyzed hydration of 2-phenylpropeneis (2004S)- a)3-phenyl-2-propanol
- b)1-phenyl-2-propanol
- c)2-phenyl-2-propanol
- d)2-phenyl-1-propanol
Correct answer is option 'C'. Can you explain this answer?
The product of acid catalyzed hydration of 2-phenylpropeneis (2004S)
a)
3-phenyl-2-propanol
b)
1-phenyl-2-propanol
c)
2-phenyl-2-propanol
d)
2-phenyl-1-propanol
|
|
Nitya Basak answered |
NOTE : Addition of water to 2-phenylpropene follows Markownikov’s rule.
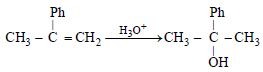
Among the following the one that gives positive iodoform test upon reaction with I2 and NaOH is [ 2006]- a)
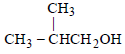
- b)PhCHOHCH3
- c)H3CH2CH(OH)CH2CH3
- d)C6H5CH2CH2OH
Correct answer is option 'B'. Can you explain this answer?
Among the following the one that gives positive iodoform test upon reaction with I2 and NaOH is [ 2006]
a)
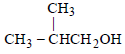
b)
PhCHOHCH3
c)
H3CH2CH(OH)CH2CH3
d)
C6H5CH2CH2OH

|
Nilesh Saini answered |
Correct Option (B) PhCHOHCH3
Explanation:
For positive iodoform test, alcohol molecule must
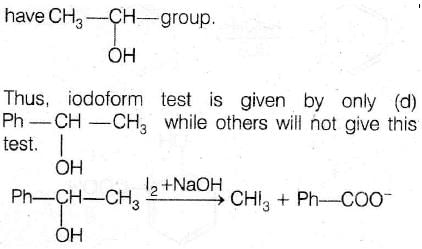
Explanation:
For positive iodoform test, alcohol molecule must

The best method to prepare cyclohexene from cyclohexanolis by using (2005S)- a)Conc. HCl + ZnCl2
- b)Conc. H3PO4
- c)HBr
- d)Conc. HCl
Correct answer is option 'B'. Can you explain this answer?
The best method to prepare cyclohexene from cyclohexanolis by using (2005S)
a)
Conc. HCl + ZnCl2
b)
Conc. H3PO4
c)
HBr
d)
Conc. HCl
|
|
Sparsh Rane answered |
TIPS/Formulae :
Conc. HCl, HBr and conc. HCl + ZnCl2 all are
nucleophiles, thus convert alcohols to alkyl halides.
However, conc. H3PO4 is a good dehydrating agent which converts an alcohol to an alkene.
Conc. HCl, HBr and conc. HCl + ZnCl2 all are
nucleophiles, thus convert alcohols to alkyl halides.
However, conc. H3PO4 is a good dehydrating agent which converts an alcohol to an alkene.
The compound which reacts fastest with Lucas reagent atroom temperature is (1981 - 1 Mark)- a)butan-1-ol
- b)butan-2-ol
- c)2-methylpropan-1-ol
- d)2-methylpropan-2-ol
Correct answer is option 'D'. Can you explain this answer?
The compound which reacts fastest with Lucas reagent atroom temperature is (1981 - 1 Mark)
a)
butan-1-ol
b)
butan-2-ol
c)
2-methylpropan-1-ol
d)
2-methylpropan-2-ol
|
|
Pranjal Saini answered |
Explanation:
The Lucas reagent is a mixture of concentrated hydrochloric acid (HCl) and zinc chloride (ZnCl2). It is used to test for the presence of primary, secondary, and tertiary alcohols.
The reaction of alcohols with Lucas reagent involves the substitution of the hydroxyl group (-OH) with a chloride ion (-Cl). The rate of this reaction depends on the stability of the carbocation intermediate formed during the reaction. Tertiary carbocations are more stable than secondary carbocations, which are more stable than primary carbocations.
Comparing the given options:
a) Butan-1-ol: This is a primary alcohol. The carbocation intermediate formed during the reaction is a primary carbocation. Primary carbocations are the least stable among the three types. Therefore, the reaction is expected to be slow.
b) Butan-2-ol: This is a secondary alcohol. The carbocation intermediate formed during the reaction is a secondary carbocation. Secondary carbocations are more stable than primary carbocations but less stable than tertiary carbocations. Therefore, the reaction is expected to be faster than butan-1-ol but slower than 2-methylpropan-2-ol.
c) 2-Methylpropan-1-ol: This is a primary alcohol. Similar to butan-1-ol, the carbocation intermediate formed during the reaction is a primary carbocation. Therefore, the reaction is expected to be slow.
d) 2-Methylpropan-2-ol: This is a tertiary alcohol. The carbocation intermediate formed during the reaction is a tertiary carbocation. Tertiary carbocations are the most stable among the three types. Therefore, the reaction is expected to be the fastest among the given options.
Conclusion:
Based on the stability of the carbocation intermediates formed during the reaction with Lucas reagent, the compound that reacts fastest at room temperature is 2-methylpropan-2-ol (option D).
The Lucas reagent is a mixture of concentrated hydrochloric acid (HCl) and zinc chloride (ZnCl2). It is used to test for the presence of primary, secondary, and tertiary alcohols.
The reaction of alcohols with Lucas reagent involves the substitution of the hydroxyl group (-OH) with a chloride ion (-Cl). The rate of this reaction depends on the stability of the carbocation intermediate formed during the reaction. Tertiary carbocations are more stable than secondary carbocations, which are more stable than primary carbocations.
Comparing the given options:
a) Butan-1-ol: This is a primary alcohol. The carbocation intermediate formed during the reaction is a primary carbocation. Primary carbocations are the least stable among the three types. Therefore, the reaction is expected to be slow.
b) Butan-2-ol: This is a secondary alcohol. The carbocation intermediate formed during the reaction is a secondary carbocation. Secondary carbocations are more stable than primary carbocations but less stable than tertiary carbocations. Therefore, the reaction is expected to be faster than butan-1-ol but slower than 2-methylpropan-2-ol.
c) 2-Methylpropan-1-ol: This is a primary alcohol. Similar to butan-1-ol, the carbocation intermediate formed during the reaction is a primary carbocation. Therefore, the reaction is expected to be slow.
d) 2-Methylpropan-2-ol: This is a tertiary alcohol. The carbocation intermediate formed during the reaction is a tertiary carbocation. Tertiary carbocations are the most stable among the three types. Therefore, the reaction is expected to be the fastest among the given options.
Conclusion:
Based on the stability of the carbocation intermediates formed during the reaction with Lucas reagent, the compound that reacts fastest at room temperature is 2-methylpropan-2-ol (option D).
A compound that gives a positive iodoform test is(1982 - 1 Mark)- a)1-pentanol
- b)2-pentanone
- c)3-pentanone
- d)pentanal
Correct answer is option 'B'. Can you explain this answer?
A compound that gives a positive iodoform test is(1982 - 1 Mark)
a)
1-pentanol
b)
2-pentanone
c)
3-pentanone
d)
pentanal
|
|
Sahaja Reddy answered |
2-pentatone becoz only an acyl group can give positive iodoform test
The correct combination of names for isomeric alcohols withmolecular formula C4H10O is/are (JEE Adv. 2014)- a)Tert-butanol and 2-methylpropan-2-ol
- b)Tert-butanol and 1, 1-dimethylethan-1-ol
- c)n-butanol and butan-1-ol
- d)Isobutyl alcohol and 2-methylpropan-1-ol
Correct answer is option 'A,C,D'. Can you explain this answer?
The correct combination of names for isomeric alcohols withmolecular formula C4H10O is/are (JEE Adv. 2014)
a)
Tert-butanol and 2-methylpropan-2-ol
b)
Tert-butanol and 1, 1-dimethylethan-1-ol
c)
n-butanol and butan-1-ol
d)
Isobutyl alcohol and 2-methylpropan-1-ol

|
Aravind Nambiar answered |
Isomeric alcohols with molecular formula C4H10O

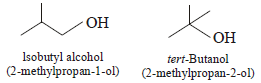
The increasing order of boiling points of the below mentioned alcohols is (2006 - 3M, –1)
(I) 1,2-dihydroxybenzene
(II) 1,3-dihydroxybenzene
(III) 1,4-dihydroxybenzene
(IV) Hydroxybenzene- a)I < II < IV < III
- b)I < II < III < IV
- c)IV < II < I < III
- d)IV < I < II < III
Correct answer is option 'D'. Can you explain this answer?
The increasing order of boiling points of the below mentioned alcohols is (2006 - 3M, –1)
(I) 1,2-dihydroxybenzene
(II) 1,3-dihydroxybenzene
(III) 1,4-dihydroxybenzene
(IV) Hydroxybenzene
(I) 1,2-dihydroxybenzene
(II) 1,3-dihydroxybenzene
(III) 1,4-dihydroxybenzene
(IV) Hydroxybenzene
a)
I < II < IV < III
b)
I < II < III < IV
c)
IV < II < I < III
d)
IV < I < II < III
|
|
User1971744 answered |
Hey due to intra h bonding ortho minimum and due to molecular mass 4 has least
An industrial method of preparation of methanol is : (1984 - 1 Mark)- a)catalytic reduction of carbon monoxide in presence ofZnO–Cr2O3
- b)by reacting methane with steam at 900ºC with a nickelcatalyst
- c)by reducing formaldehyde with lithium aluminiumhydride
- d)by reacting formaldehyde with aqueous sodiumhydroxide solution
Correct answer is option 'A'. Can you explain this answer?
An industrial method of preparation of methanol is : (1984 - 1 Mark)
a)
catalytic reduction of carbon monoxide in presence ofZnO–Cr2O3
b)
by reacting methane with steam at 900ºC with a nickelcatalyst
c)
by reducing formaldehyde with lithium aluminiumhydride
d)
by reacting formaldehyde with aqueous sodiumhydroxide solution
|
|
Siddharth Rane answered |
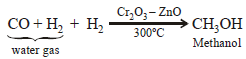
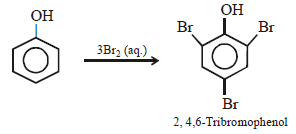
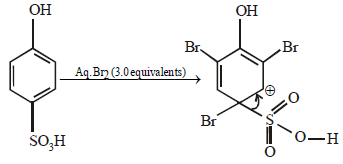
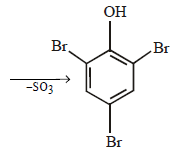
When phenol is treated with excess bromine water, it gives: (1984 - 1 Mark)- a)m-Bromophenol
- b)o- and p-Bromophenol
- c)2, 4-Dibromophenol
- d)2, 4, 6-Tribromophenol
Correct answer is option 'D'. Can you explain this answer?
When phenol is treated with excess bromine water, it gives: (1984 - 1 Mark)
a)
m-Bromophenol
b)
o- and p-Bromophenol
c)
2, 4-Dibromophenol
d)
2, 4, 6-Tribromophenol
|
|
Akanksha Nair answered |
NOTE :
The –OH group in phenol, being activating group, facilitates substitution in the o- and p-positions.
The –OH group in phenol, being activating group, facilitates substitution in the o- and p-positions.
p -cresol reacts with chloroform in alkaline medium to give the compound A which adds hydrogen cyanide to form, the
compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is
[2005]- a)
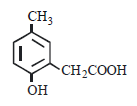
- b)
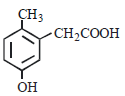
- c)
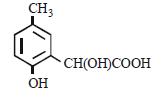
- d)
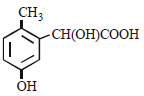
Correct answer is option 'C'. Can you explain this answer?
p -cresol reacts with chloroform in alkaline medium to give the compound A which adds hydrogen cyanide to form, the
compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is
[2005]
compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is
[2005]
a)

b)

c)

d)

|
|
Akash Kumar answered |

From amongst the following alcohols the one that wouldreact fastest with conc. HCl and anhydrous ZnCl2, is [2010]- a)2-Butanol
- b)2- Methylpropan-2-ol
- c)2-Methylpropanol
- d)1- Butanol
Correct answer is option 'B'. Can you explain this answer?
From amongst the following alcohols the one that wouldreact fastest with conc. HCl and anhydrous ZnCl2, is [2010]
a)
2-Butanol
b)
2- Methylpropan-2-ol
c)
2-Methylpropanol
d)
1- Butanol
|
|
Ishan Chatterjee answered |
Reactivity of Alcohols with Concentrated HCl and Anhydrous ZnCl2
When alcohols react with concentrated hydrochloric acid (HCl) and anhydrous zinc chloride (ZnCl2), the alcohol undergoes an elimination reaction to form an alkene. This reaction is known as the Lucas test and is used to differentiate between primary, secondary, and tertiary alcohols based on their reactivity.
The Lucas Test
The Lucas test is based on the principle that the rate of reaction between an alcohol and HCl/ZnCl2 mixture depends on the structure of the alcohol. The reactivity order for alcohols in this test is as follows:
1. Tertiary alcohols react the fastest.
2. Secondary alcohols react moderately.
3. Primary alcohols react the slowest.
Explanation of the Answer
Among the given options, the correct answer is (b) 2-Methylpropan-2-ol. Let's break down the structure of each option and analyze their reactivity.
a) 2-Butanol
- It is a primary alcohol.
- It has four carbon atoms with the hydroxyl group attached to the second carbon.
- The reaction with HCl/ZnCl2 will be relatively slow compared to the other options.
b) 2-Methylpropan-2-ol
- It is a tertiary alcohol.
- It has four carbon atoms with a methyl group attached to the second carbon and a hydroxyl group attached to the third carbon.
- Tertiary alcohols react the fastest in the Lucas test.
- Therefore, it is the most reactive among the given options.
c) 2-Methylpropanol
- It is a secondary alcohol.
- It has three carbon atoms with a methyl group attached to the second carbon and a hydroxyl group attached to the first carbon.
- Secondary alcohols react moderately in the Lucas test.
- It is less reactive than option (b) but more reactive than option (a).
d) 1-Butanol
- It is a primary alcohol.
- It has four carbon atoms with the hydroxyl group attached to the first carbon.
- It will have the slowest reaction among the given options.
Conclusion
Based on the reactivity order in the Lucas test, the alcohol that will react fastest with concentrated HCl and anhydrous ZnCl2 is 2-Methylpropan-2-ol (option b). Tertiary alcohols generally have the highest reactivity in elimination reactions, followed by secondary alcohols, while primary alcohols react the slowest.
When alcohols react with concentrated hydrochloric acid (HCl) and anhydrous zinc chloride (ZnCl2), the alcohol undergoes an elimination reaction to form an alkene. This reaction is known as the Lucas test and is used to differentiate between primary, secondary, and tertiary alcohols based on their reactivity.
The Lucas Test
The Lucas test is based on the principle that the rate of reaction between an alcohol and HCl/ZnCl2 mixture depends on the structure of the alcohol. The reactivity order for alcohols in this test is as follows:
1. Tertiary alcohols react the fastest.
2. Secondary alcohols react moderately.
3. Primary alcohols react the slowest.
Explanation of the Answer
Among the given options, the correct answer is (b) 2-Methylpropan-2-ol. Let's break down the structure of each option and analyze their reactivity.
a) 2-Butanol
- It is a primary alcohol.
- It has four carbon atoms with the hydroxyl group attached to the second carbon.
- The reaction with HCl/ZnCl2 will be relatively slow compared to the other options.
b) 2-Methylpropan-2-ol
- It is a tertiary alcohol.
- It has four carbon atoms with a methyl group attached to the second carbon and a hydroxyl group attached to the third carbon.
- Tertiary alcohols react the fastest in the Lucas test.
- Therefore, it is the most reactive among the given options.
c) 2-Methylpropanol
- It is a secondary alcohol.
- It has three carbon atoms with a methyl group attached to the second carbon and a hydroxyl group attached to the first carbon.
- Secondary alcohols react moderately in the Lucas test.
- It is less reactive than option (b) but more reactive than option (a).
d) 1-Butanol
- It is a primary alcohol.
- It has four carbon atoms with the hydroxyl group attached to the first carbon.
- It will have the slowest reaction among the given options.
Conclusion
Based on the reactivity order in the Lucas test, the alcohol that will react fastest with concentrated HCl and anhydrous ZnCl2 is 2-Methylpropan-2-ol (option b). Tertiary alcohols generally have the highest reactivity in elimination reactions, followed by secondary alcohols, while primary alcohols react the slowest.
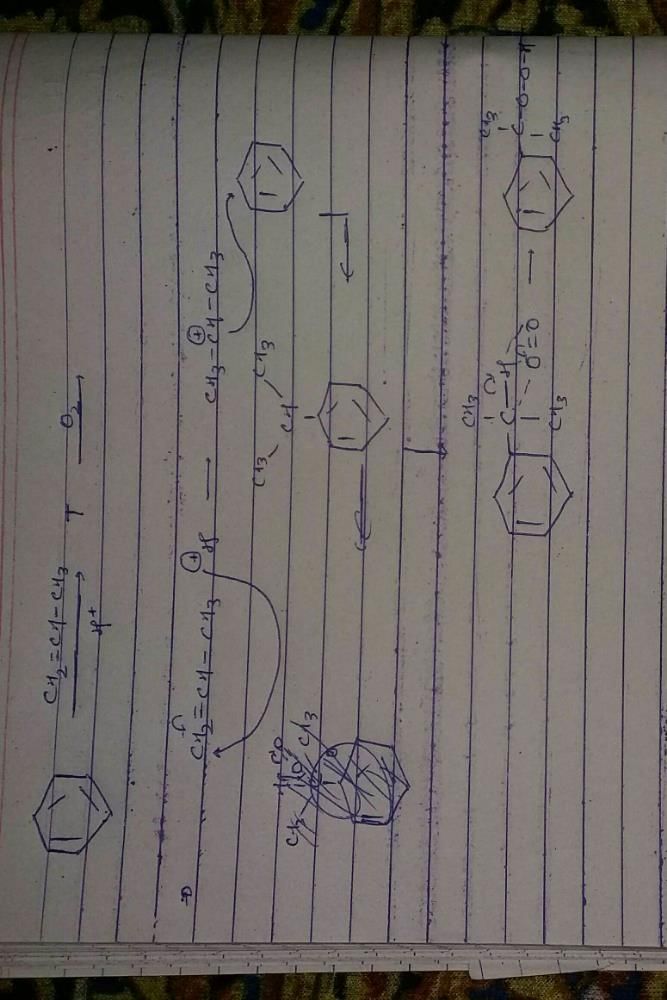
The main product of the following reaction is
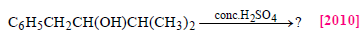
- a)
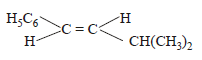
- b)
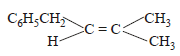
- c)
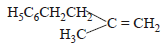
- d)
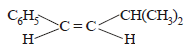
Correct answer is option 'A'. Can you explain this answer?
The main product of the following reaction is
a)
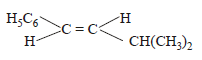
b)
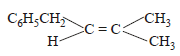
c)
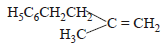
d)
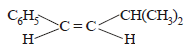
|
|
Maulik Saha answered |
Whenever dehydration can produce two different alkenes, major product is formed according to Saytzeff rule i.e. more substituted alkene (alkene having lesser
number of hydrogen atoms on the two doubly bonded carbon atoms) is the major product.
Such reactions which can produce two or more structural isomers but one of them in greater amounts than the other are called regioselective ; in case a reaction is 100% regioselective, it is termed as regiospecific.
In addition to being regioselective, alcohol
dehydrations are stereoselective (a reaction in which a single starting material can yield two or more stereoisomeric products, but gives one of them in greater amount than any other).
number of hydrogen atoms on the two doubly bonded carbon atoms) is the major product.
Such reactions which can produce two or more structural isomers but one of them in greater amounts than the other are called regioselective ; in case a reaction is 100% regioselective, it is termed as regiospecific.
In addition to being regioselective, alcohol
dehydrations are stereoselective (a reaction in which a single starting material can yield two or more stereoisomeric products, but gives one of them in greater amount than any other).
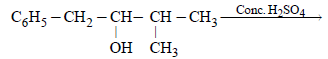
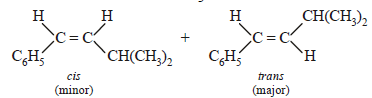
Which of the following is basic- a)CH3 – CH2 – OH
- b)OH – CH2 – CH2 – OH
- c)H – O – O – H
- d)
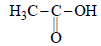
Correct answer is option 'A'. Can you explain this answer?
Which of the following is basic
a)
CH3 – CH2 – OH
b)
OH – CH2 – CH2 – OH
c)
H – O – O – H
d)
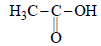

|
Aqsa Kibria answered |
Firstly alcohols are most basic among all oxygen compounds. now secondly It is the fact that alkyl grps have effect that means it will tend to give its electron towards the oxygen atom on account of increase in electron density on oxygen atom it given its one elctron pair and behaves as a base.
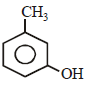
The structure of the compound that gives a tribromo derivative on treatment with bromine water is [2006]- a)
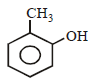
- b)
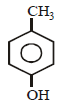
- c)
- d)
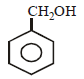
Correct answer is option 'C'. Can you explain this answer?
The structure of the compound that gives a tribromo derivative on treatment with bromine water is [2006]
a)

b)

c)

d)

|
|
Niharika Deshpande answered |
NOTE : OH group activates the benzene nucleus and
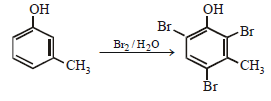
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields: [JEE M 2016]
(i) 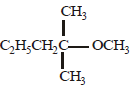 (ii)
(ii) 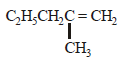 (iii)
(iii)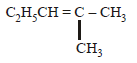
- a)(3) only
- b)(a) and (b)
- c)All of these
- d)(a) and (c)
Correct answer is option 'A'. Can you explain this answer?
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields: [JEE M 2016]
(i)
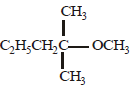
(ii)
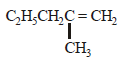
(iii)
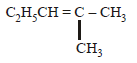
a)
(3) only
b)
(a) and (b)
c)
All of these
d)
(a) and (c)

|
Lakshay Kumar answered |
Refer saytzeff rule...double bond faormation takes place in most suitable place,that's why 2 doesn't come
HBr reacts fastest with : (1986 - 1 Mark)- a)2-methylpropan-2-ol
- b)propan-1-ol
- c)propan-2-ol
- d)2-methylpropan-1-ol
Correct answer is option 'A'. Can you explain this answer?
HBr reacts fastest with : (1986 - 1 Mark)
a)
2-methylpropan-2-ol
b)
propan-1-ol
c)
propan-2-ol
d)
2-methylpropan-1-ol
|
|
Kirti Desai answered |
Reactions involving cleavage of carbon-oxygen bond, (C – OH) follows the following order :
Tertiary > Secondary > Primary
Tertiary > Secondary > Primary
HBr reacts with CH2 = CH – OCH3 under anhydrous conditions at room temperature to give [ 2006]- a)BrCH2 – CH2 – OCH3
- b)H3C – CHBr – OCH3
- c)CH3CHO and CH3Br
- d)BrCH2CHO and CH3OH
Correct answer is option 'B'. Can you explain this answer?
HBr reacts with CH2 = CH – OCH3 under anhydrous conditions at room temperature to give [ 2006]
a)
BrCH2 – CH2 – OCH3
b)
H3C – CHBr – OCH3
c)
CH3CHO and CH3Br
d)
BrCH2CHO and CH3OH
|
|
Gopal Verma answered |
Methyl vinyl ether under anhydrous condition at room temperature undergoes addition reaction.
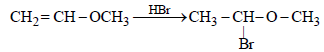
The reactivity of compound Z with different halogens under appropriate conditions is given below: (JEE Adv. 2014)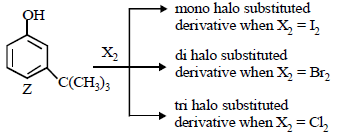 The observed pattern of electrophilic substitution can be explained by
The observed pattern of electrophilic substitution can be explained by- a)The steric effect of the halogen
- b)The steric effect of the tert-butyl group
- c)The electronic effect of the phenolic group
- d)The electronic effect of the tert-butyl group
Correct answer is option 'A,B,C'. Can you explain this answer?
The reactivity of compound Z with different halogens under appropriate conditions is given below: (JEE Adv. 2014)

The observed pattern of electrophilic substitution can be explained by
a)
The steric effect of the halogen
b)
The steric effect of the tert-butyl group
c)
The electronic effect of the phenolic group
d)
The electronic effect of the tert-butyl group
|
|
Baishali Sarkar answered |
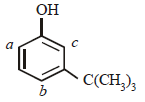
—OH group is strongly activating and o, p-directing due to +M effect. Thus positions a, b and c are the sites for attack by an electrophile. However, sites b and c are not preferred by bulky electrophile due to steric crowding. Thus more bulky electrophile (like I2) can attack only site a, which is least sterically hindered, a bit smaller electrophile (Br2) can attack at sites a and also b (relatively less sterically hindered site) and the smallest electrophile (Cl2) can attack all the three sites, viz., a, b and c (most sterically hindered site).
The reaction of  with HBr gives (1998 - 2 Marks)
with HBr gives (1998 - 2 Marks)- a)
- b)
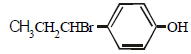
- c)
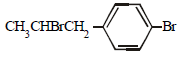
- d)
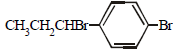
Correct answer is option 'B'. Can you explain this answer?
The reaction of  with HBr gives (1998 - 2 Marks)
with HBr gives (1998 - 2 Marks)
 with HBr gives (1998 - 2 Marks)
with HBr gives (1998 - 2 Marks)a)
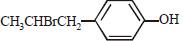
b)

c)
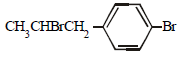
d)

|
|
Vivek answered |
Markownikoff addition. Negative part of the reagent goes to the carbon with the lesser number of hydrogen atoms.
The compound that will react most readily with NaOH toform methanol is (2001S)- a)(CH3)4N+I-
- b)CH3OCH3
- c)(CH3 )3S+I-
- d)(CH3)3CCl
Correct answer is option 'A'. Can you explain this answer?
The compound that will react most readily with NaOH toform methanol is (2001S)
a)
(CH3)4N+I-
b)
CH3OCH3
c)
(CH3 )3S+I-
d)
(CH3)3CCl
|
|
Sravya Nair answered |
TIPS/Formulae :
Compound (CH3)4N+I– is most reactive due to (i) better
leaving group, I– and (ii) due to the fact that the methyl group, with positive N, is more electron deficient.
Hence this group is more reactive towards nucleophile, OH–
Compound (CH3)4N+I– is most reactive due to (i) better
leaving group, I– and (ii) due to the fact that the methyl group, with positive N, is more electron deficient.
Hence this group is more reactive towards nucleophile, OH–
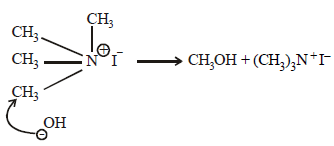
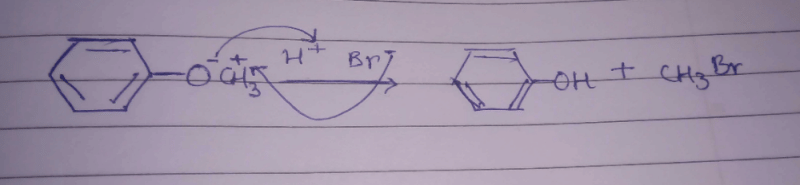
The ether 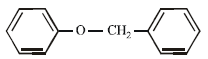 when treated with HI produces (1999 - 3 Marks)
when treated with HI produces (1999 - 3 Marks)- a)
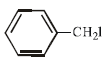
- b)
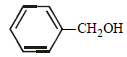
- c)
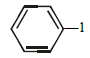
- d)
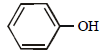
Correct answer is option 'A,D'. Can you explain this answer?
The ether 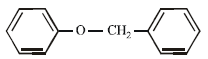 when treated with HI produces (1999 - 3 Marks)
when treated with HI produces (1999 - 3 Marks)
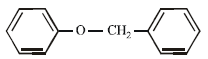 when treated with HI produces (1999 - 3 Marks)
when treated with HI produces (1999 - 3 Marks)a)
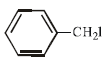
b)
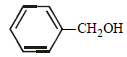
c)

d)

|
|
Vivek answered |
Here the main thing is to decide which bond to break. The bond between O and CH2 will be broken because this will form PHENOXIDE ion which is highly stable. Bond between O and benzene ring won't be broken as in that case, PHENYL carbocation would be formed which is highly unstable. So C6H5—O bond won't be broken.
Now, PHENOXIDE ion will get the H+ and BENZYL carbocation will get I-. So, A and D will be formed.
Upvote agr answer pasand aaye 😁😁😁😁🙂
Phenol reacts with bromine in carbon disulphide at lowtemperature to give (1988 - 1 Mark)- a)m-bromophenol
- b)o- and p-bromophenol
- c)p-bromophenol
- d)2, 4, 6-tribromophenol
Correct answer is option 'B'. Can you explain this answer?
Phenol reacts with bromine in carbon disulphide at lowtemperature to give (1988 - 1 Mark)
a)
m-bromophenol
b)
o- and p-bromophenol
c)
p-bromophenol
d)
2, 4, 6-tribromophenol
|
|
Sravya Nair answered |
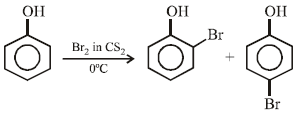

NOTE : In absence of CS2, polyhalogenation in o- and p-positions takes place.
In the reaction 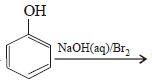 the intermediate (s) is (are) (2010)
the intermediate (s) is (are) (2010)- a)
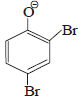
- b)
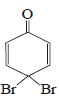
- c)
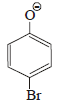
- d)
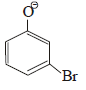
Correct answer is option 'A,C'. Can you explain this answer?
In the reaction  the intermediate (s) is (are) (2010)
the intermediate (s) is (are) (2010)
 the intermediate (s) is (are) (2010)
the intermediate (s) is (are) (2010)a)

b)

c)

d)


|
Akshat Chavan answered |
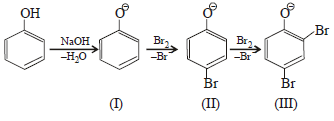
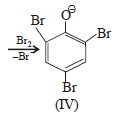
Product of reaction of phenol with NaOH/Br2 is sodium salt of 2,4,6-tribromophenol. Hence, species (I), (II), (III) are formed as intermediate.
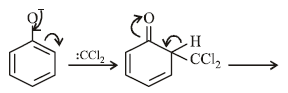
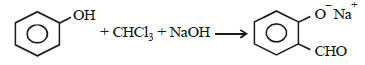 The electrophile involved in the above reaction is
The electrophile involved in the above reaction is- a)trichloromethyl anion
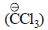
- b)formyl cation

- c)dichloromethyl cation
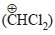
- d)dichlorocarbene (: CCl2)
Correct answer is option 'D'. Can you explain this answer?
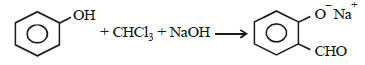
The electrophile involved in the above reaction is
a)
trichloromethyl anion 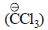
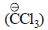
b)
formyl cation 

c)
dichloromethyl cation 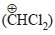
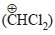
d)
dichlorocarbene (: CCl2)
|
|
Yash Modi answered |
The above reaction is the Reimer Tiemann rxn. where singlet carbenes are the reaction intermediate.
1-Propanol and 2-propanol can be best distinguished by (2001S)- a)oxidation with alkaline KMnO4 followed by reactionwith Fehling solution
- b)oxidation with acidic dichromate followed by reactionwith Fehling solution
- c)oxidation by heating with copper followed by reactionwith Fehling solution
- d)oxidation with concentrated H2SO4 followed byreaction with Fehling solution
Correct answer is option 'C'. Can you explain this answer?
1-Propanol and 2-propanol can be best distinguished by (2001S)
a)
oxidation with alkaline KMnO4 followed by reactionwith Fehling solution
b)
oxidation with acidic dichromate followed by reactionwith Fehling solution
c)
oxidation by heating with copper followed by reactionwith Fehling solution
d)
oxidation with concentrated H2SO4 followed byreaction with Fehling solution
|
|
Anirban Banerjee answered |
NOTE :
Fehling solution is a weak oxidising agent which can oxidise aldehyde but not ketone.
Primary alcohols undergoes oxidation with alkaline KMnO4, acidic dichromate and conc. H2SO4 to give acids, whereas with Cu they give aldehydes.
Fehling solution is a weak oxidising agent which can oxidise aldehyde but not ketone.
Primary alcohols undergoes oxidation with alkaline KMnO4, acidic dichromate and conc. H2SO4 to give acids, whereas with Cu they give aldehydes.
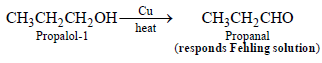
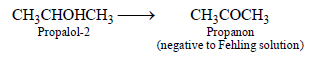
Ethyl alcohol is heated with conc H2SO4 the product formed is- a)
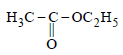
- b)C2H6
- c)C2H4
- d)C2H2
Correct answer is option 'C'. Can you explain this answer?
Ethyl alcohol is heated with conc H2SO4 the product formed is
a)
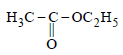
b)
C2H6
c)
C2H4
d)
C2H2
|
|
Vivek answered |
This reaction is ACID CATALYSED DEHYDRATION OF ALCOHOL. In this water will be removed from alcohol leaving behind CH2=CH2
Among the following compounds which can be dehydrated very easily is [2004]- a)
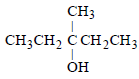
- b)
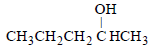
- c)CH3CH2CH2CH2CH2OH
- d)
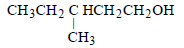
Correct answer is option 'A'. Can you explain this answer?
Among the following compounds which can be dehydrated very easily is [2004]
a)
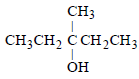
b)
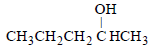
c)
CH3CH2CH2CH2CH2OH
d)
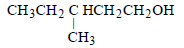
|
|
Smruti Sucharita answered |
tertiary carbocation has more tendency for dehydration ..so optn a is correct
For the identification of b-naphthol using dye test, it isnecessary to use (JEE Adv. 2014)- a)Dichloromethane solution of b-naphthol
- b)Acidic solution of b-naphthol
- c)Neutral solution of b-naphthol
- d)Alkaline solution of b-naphthol
Correct answer is option 'D'. Can you explain this answer?
For the identification of b-naphthol using dye test, it isnecessary to use (JEE Adv. 2014)
a)
Dichloromethane solution of b-naphthol
b)
Acidic solution of b-naphthol
c)
Neutral solution of b-naphthol
d)
Alkaline solution of b-naphthol
|
|
Ashutosh Shah answered |
In dye test, phenolic — OH group is converted to — O– which activates the ring towards electrophilic aromatic substitution
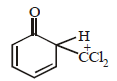
When phenol is reacted with CHCl3 and NaOH followed by acidification, salicyladehyde is obtained. Which of the
following species are involved in the above mentioned reaction as intermediate?- a)
- b)
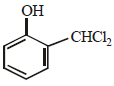
- c)
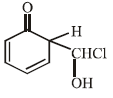
- d)
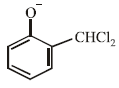
Correct answer is option 'D'. Can you explain this answer?
When phenol is reacted with CHCl3 and NaOH followed by acidification, salicyladehyde is obtained. Which of the
following species are involved in the above mentioned reaction as intermediate?
following species are involved in the above mentioned reaction as intermediate?
a)

b)

c)

d)

|
|
Bhavya Kumar answered |
TIPS/Formulae :
Riemer-Tiemann reaction involves electrophilic
substitution on the highly reactive phenoxide ring.
Riemer-Tiemann reaction involves electrophilic
substitution on the highly reactive phenoxide ring.
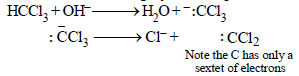
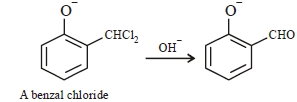
An unknown alcohol is treated with the “Lucas reagent” todetermine whether the alcohol is primary, secondary ortertiary. Which alcohol reacts fastest and by what mechanism:[JEE M 2013]- a)secondary alcohol by SN1
- b)tertiary alcohol by SN1
- c)secondary alcohol by SN2
- d)tertiary alcohol by SN2
Correct answer is option 'B'. Can you explain this answer?
An unknown alcohol is treated with the “Lucas reagent” todetermine whether the alcohol is primary, secondary ortertiary. Which alcohol reacts fastest and by what mechanism:[JEE M 2013]
a)
secondary alcohol by SN1
b)
tertiary alcohol by SN1
c)
secondary alcohol by SN2
d)
tertiary alcohol by SN2
|
|
Vivek answered |
Tertiary alcohol reacts fastest with Lucas Reagent by Sn1 mechanism. (immediately)
Now, Sn1 bcoz their is high steric hindrance in tertiary alcohol which will not be favoured if it reacts by Sn2 mechanism. Furthermore, the tertiary carbocation is very stable. So, its formation is favoured.
😎😎😎😎
Chapter doubts & questions for Alcohols, Phenols and Ethers - 35 Years Chapter wise Previous Year Solved Papers for JEE 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Alcohols, Phenols and Ethers - 35 Years Chapter wise Previous Year Solved Papers for JEE in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup


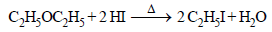

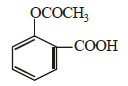
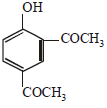
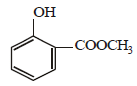
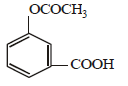
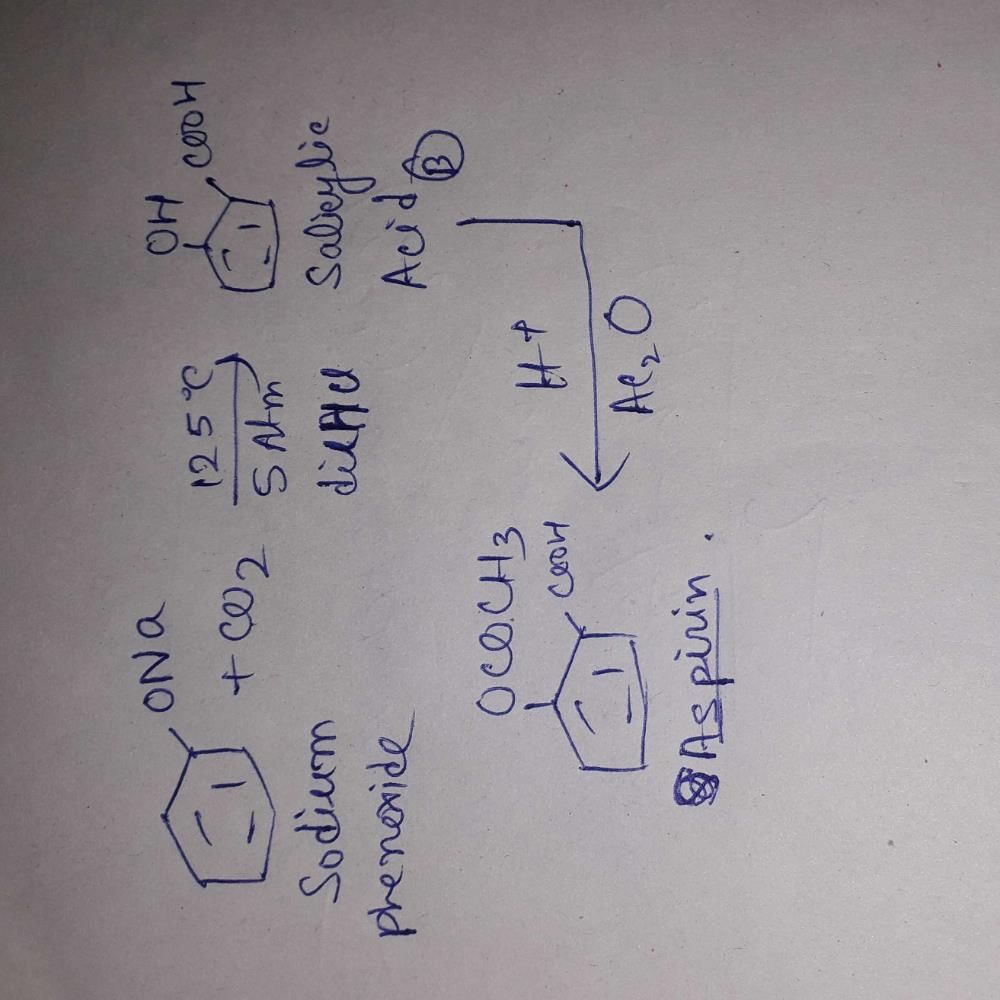


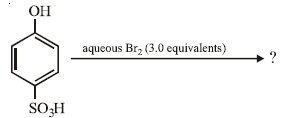
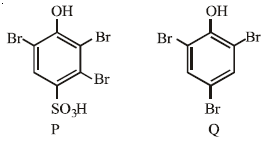
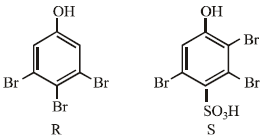
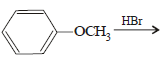 the products are
the products are