All Exams >
JEE >
35 Years Chapter wise Previous Year Solved Papers for JEE >
All Questions
All questions of Electrochemistry for JEE Exam
For a spontaneous reaction the DG, equilibrium constant (K) and 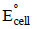 will be respectively [2005]
will be respectively [2005]- a)–ve, >1, –ve
- b)–ve, <1, –ve
- c)+ve, >1, –ve
- d)–ve, >1, +ve
Correct answer is option 'D'. Can you explain this answer?
For a spontaneous reaction the DG, equilibrium constant (K) and 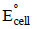 will be respectively [2005]
will be respectively [2005]
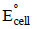 will be respectively [2005]
will be respectively [2005]a)
–ve, >1, –ve
b)
–ve, <1, –ve
c)
+ve, >1, –ve
d)
–ve, >1, +ve
|
|
Krishna Iyer answered |
NOTE : For spontaneous reaction DG should be negative. Equilibrium constant should be more than one
(ΔG = – 2.303 RT log Kc, If Kc = 1 then ΔG = 0; If Kc < 1
EMF of a cell in terms of reduction potential of its left andright electrodes is [2002]- a)E = Eleft - Eright
- b)E = Eleft + Eright
- c)E = Eright - Eleft
- d)E = – (Eright + Eleft).
Correct answer is option 'C'. Can you explain this answer?
EMF of a cell in terms of reduction potential of its left andright electrodes is [2002]
a)
E = Eleft - Eright
b)
E = Eleft + Eright
c)
E = Eright - Eleft
d)
E = – (Eright + Eleft).
|
|
Geetika Shah answered |
Ecell = Reduction potential of cathode (right)
– Reduction potential of anode (left)
= Eright – Eleft.
– Reduction potential of anode (left)
= Eright – Eleft.
The standard e.m.f. of a cell involving one electron changeis found to be 0.591 V at 25ºC. The equilibrium constant ofthe reaction is (F = 96,500 C mol–1; R = 8.314 JK–1 mol–1) [2004]- a)1.0 × 1010
- b)1.0 × 105
- c)1.0 × 101
- d)1.0 × 1030
Correct answer is option 'A'. Can you explain this answer?
The standard e.m.f. of a cell involving one electron changeis found to be 0.591 V at 25ºC. The equilibrium constant ofthe reaction is (F = 96,500 C mol–1; R = 8.314 JK–1 mol–1) [2004]
a)
1.0 × 1010
b)
1.0 × 105
c)
1.0 × 101
d)
1.0 × 1030
|
|
Tarun Mahawar answered |
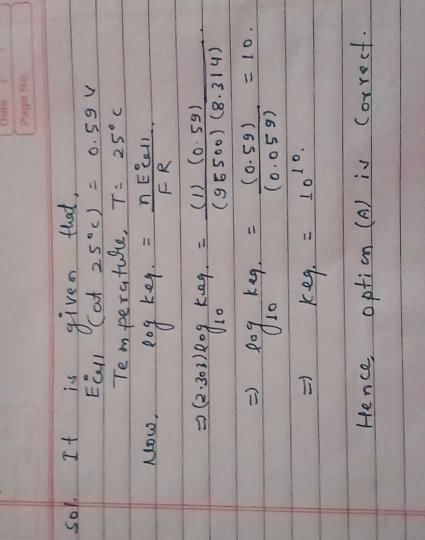
Several blocks of magnesium are fixed to the bottom of a ship to [2003]- a)make the ship lighter
- b)prevent action of water and salt
- c)prevent puncturing by under-sea rocks
- d)keep away the sharks
Correct answer is option 'A'. Can you explain this answer?
Several blocks of magnesium are fixed to the bottom of a ship to [2003]
a)
make the ship lighter
b)
prevent action of water and salt
c)
prevent puncturing by under-sea rocks
d)
keep away the sharks

|
Vikas Saini answered |
Right answer is B,as Mg is used to prevent corrison of Iron by sea water.ye haramii course creator ek bar course bana k bath jate h,uske bad koi bhi error correct nhi karte h Baddve salee
Standard reduction electrode potentials of three metals A, B& C are respectively + 0.5 V, – 3.0 V & –1.2 V. The reducingpowers of these metals are [2003]- a)A > B > C
- b)C > B > A
- c)A > C > B
- d)B > C > A
Correct answer is option 'D'. Can you explain this answer?
Standard reduction electrode potentials of three metals A, B& C are respectively + 0.5 V, – 3.0 V & –1.2 V. The reducingpowers of these metals are [2003]
a)
A > B > C
b)
C > B > A
c)
A > C > B
d)
B > C > A
|
|
Anand Kumar answered |
Reduction potential and oxidising nature are same thing and on the other side oxidation potential and reducing nature are same thing.
So, reduction potential increases then oxidising nature increases but oxidation potential and reducing nature decreases
so, answer is B>C>A
Note:- more +ve reduction potential implies high oxidising nature.
So, reduction potential increases then oxidising nature increases but oxidation potential and reducing nature decreases
so, answer is B>C>A
Note:- more +ve reduction potential implies high oxidising nature.
Consider the following cell reaction: (2011)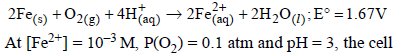 potential at 25ºC is
potential at 25ºC is- a)1.47 V
- b)1.77 V
- c)1.87 V
- d)1.57 V
Correct answer is option 'D'. Can you explain this answer?
Consider the following cell reaction: (2011)
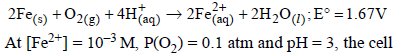
potential at 25ºC is
a)
1.47 V
b)
1.77 V
c)
1.87 V
d)
1.57 V
|
|
Lavanya Menon answered |
Here n = 4, and [H+] = 10–pH = 10–3
Applying Nernst equation
Applying Nernst equation
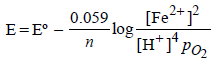
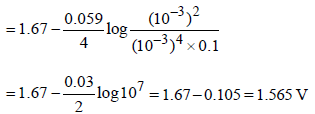
Given :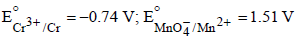
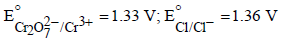 Based on the data given above, strongest oxidising agentwill be : [JEE M 2013]
Based on the data given above, strongest oxidising agentwill be : [JEE M 2013]- a)Cl
- b)Cr3+
- c)Mn2+
- d)
Correct answer is option 'D'. Can you explain this answer?
Given :
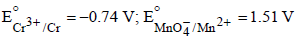
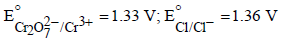
Based on the data given above, strongest oxidising agentwill be : [JEE M 2013]
a)
Cl
b)
Cr3+
c)
Mn2+
d)
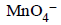
|
|
Harsh Singhal answered |
Mno4- have highest reduction potential so strongest oxidising agent
For the reduction of  ion in an aqueous solution, E° is + 0.96V. Values of E° for some metal ions are given below
ion in an aqueous solution, E° is + 0.96V. Values of E° for some metal ions are given below
V2+ (aq) + 2e– → V E° = – 1.19 V
Fe3+ (aq) + 3e– → Fe E° = – 0.04 V
Au3+ (aq) + 3e– → Au E° = + 1.40 V
Hg2+ (aq) + 2e– →Hg E° = + 0.86 VThe pair(s) of metals that is(are) oxidized by  inaqueous solution is(are) (2009)
inaqueous solution is(are) (2009)- a)V and Hg
- b)Hg and Fe
- c)Fe and Au
- d)Fe and V
Correct answer is option 'A,B,C'. Can you explain this answer?
For the reduction of  ion in an aqueous solution, E° is + 0.96V. Values of E° for some metal ions are given below
ion in an aqueous solution, E° is + 0.96V. Values of E° for some metal ions are given below
V2+ (aq) + 2e– → V E° = – 1.19 V
Fe3+ (aq) + 3e– → Fe E° = – 0.04 V
Au3+ (aq) + 3e– → Au E° = + 1.40 V
Hg2+ (aq) + 2e– →Hg E° = + 0.86 V
 ion in an aqueous solution, E° is + 0.96V. Values of E° for some metal ions are given below
ion in an aqueous solution, E° is + 0.96V. Values of E° for some metal ions are given belowV2+ (aq) + 2e– → V E° = – 1.19 V
Fe3+ (aq) + 3e– → Fe E° = – 0.04 V
Au3+ (aq) + 3e– → Au E° = + 1.40 V
Hg2+ (aq) + 2e– →Hg E° = + 0.86 V
The pair(s) of metals that is(are) oxidized by  inaqueous solution is(are) (2009)
inaqueous solution is(are) (2009)
 inaqueous solution is(are) (2009)
inaqueous solution is(are) (2009)a)
V and Hg
b)
Hg and Fe
c)
Fe and Au
d)
Fe and V

|
Prince Bhargav answered |
Oxidising nature is proportional to Erp ,,,ERP zyaada toh khud reduce hone ki tendency zyaada or doosro ko oxidise karne ki tendency bhi zyaada toh no3- se kam jiska erp hoga voh no3- se kam acha oxidising agent hoga so answer is a b and d
The equivalent conductances of two strong electrolytes at infinite dilution in H2O (where ions move freely through a
solution) at 25°C are given below : [2007]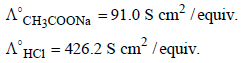 What additional information/ quantity one needs to calculate
What additional information/ quantity one needs to calculate 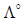 of an aqueous solution of acetic acid?
of an aqueous solution of acetic acid?- a)
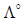 of chloroacetic acid (ClCH2COOH)
of chloroacetic acid (ClCH2COOH) - b)
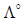 of NaCl
of NaCl - c)
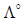 of CH3COOK
of CH3COOK - d)the limiting equivalent coductance of
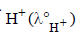
Correct answer is option 'B'. Can you explain this answer?
The equivalent conductances of two strong electrolytes at infinite dilution in H2O (where ions move freely through a
solution) at 25°C are given below : [2007]
solution) at 25°C are given below : [2007]
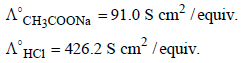
What additional information/ quantity one needs to calculate 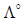 of an aqueous solution of acetic acid?
of an aqueous solution of acetic acid?
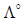 of an aqueous solution of acetic acid?
of an aqueous solution of acetic acid?a)
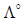 of chloroacetic acid (ClCH2COOH)
of chloroacetic acid (ClCH2COOH)b)
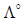 of NaCl
of NaClc)
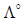 of CH3COOK
of CH3COOKd)
the limiting equivalent coductance of 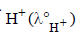
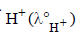
|
|
Saumya Choudhury answered |
NOTE : According to Kohlrausch’s law, molar
conductivity of weak electrolyte acetic acid (CH3COOH) can be calculated as follows:
conductivity of weak electrolyte acetic acid (CH3COOH) can be calculated as follows:
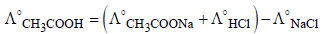
∴ Value of 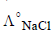 should also be known for
should also be known for
calculating value of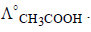
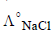 should also be known for
should also be known forcalculating value of
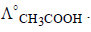
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2++ H2(g) addition of H2SO4 to cathode compartment, will [2004]- a)increase the E and shift equilibrium to the right
- b)lower the E and shift equilibrium to the right
- c)lower the E and shift equlibrium to the left
- d)increase the E and shift equilibrium to the left
Correct answer is option 'A'. Can you explain this answer?
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2++ H2(g) addition of H2SO4 to cathode compartment, will [2004]
a)
increase the E and shift equilibrium to the right
b)
lower the E and shift equilibrium to the right
c)
lower the E and shift equlibrium to the left
d)
increase the E and shift equilibrium to the left

|
Saikat Dasgupta answered |
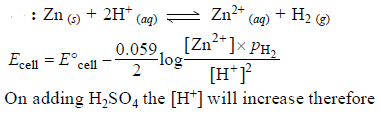
A solution containing one mole per litre of each Cu(NO3)2; AgNO3; Hg2(NO3)2; is being electrolysed by using inert
electrodes. The values of standard electrode potentials in volts (reduction potentials) are : (1984 - 1 Mark)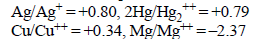 With increasing voltage, the sequence of deposition of metalson the cathode will be :
With increasing voltage, the sequence of deposition of metalson the cathode will be :- a)Ag, Hg, Cu, Mg
- b)Mg, Cu, Hg, Ag
- c)Ag, Hg, Cu
- d)Cu, Hg, Ag
Correct answer is option 'C'. Can you explain this answer?
A solution containing one mole per litre of each Cu(NO3)2; AgNO3; Hg2(NO3)2; is being electrolysed by using inert
electrodes. The values of standard electrode potentials in volts (reduction potentials) are : (1984 - 1 Mark)
electrodes. The values of standard electrode potentials in volts (reduction potentials) are : (1984 - 1 Mark)
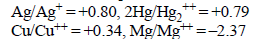
With increasing voltage, the sequence of deposition of metalson the cathode will be :
a)
Ag, Hg, Cu, Mg
b)
Mg, Cu, Hg, Ag
c)
Ag, Hg, Cu
d)
Cu, Hg, Ag

|
Bhavana Dey answered |
The reduction potentials (as given) of the ions are in the order :
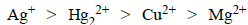
Mg2+ (aq.) will not be reduced as its reduction potential is much lower than that of water (–0.83 V). Hence the sequence of deposition of the metals will be Ag, Hg, Cu.
A gas X at 1 atm is bubbled through a solution containing amixture of 1 M Y– and M Z– at 25°C. If the reduction potentialof Z > Y > X, then, (1999 - 2 Marks)- a)Y will oxidize X and not Z
- b)Y will oxidize Z and not X
- c)Y will oxidize both X and Z
- d)Y will reduce both X and Z
Correct answer is option 'A'. Can you explain this answer?
A gas X at 1 atm is bubbled through a solution containing amixture of 1 M Y– and M Z– at 25°C. If the reduction potentialof Z > Y > X, then, (1999 - 2 Marks)
a)
Y will oxidize X and not Z
b)
Y will oxidize Z and not X
c)
Y will oxidize both X and Z
d)
Y will reduce both X and Z
|
|
Shalini Yadav answered |
The given order of reduction potentials is Z > Y > X. A
spontaneous reaction will have the following
characteristics
Z reduced and Y oxidised
Z reduced and X oxidised
Y reduced and X oxidised
Hence, Y will oxidise X and not Z.
spontaneous reaction will have the following
characteristics
Z reduced and Y oxidised
Z reduced and X oxidised
Y reduced and X oxidised
Hence, Y will oxidise X and not Z.
Which of the following reaction is possible at anode?- a)
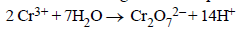
- b)F2 → 2F-
- c)(1/2) O2 + 2H+ → H2O
- d)None of these.
Correct answer is option 'A'. Can you explain this answer?
Which of the following reaction is possible at anode?
a)
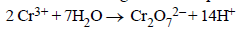
b)
F2 → 2F-
c)
(1/2) O2 + 2H+ → H2O
d)
None of these.
|
|
Vivek answered |
At Anode oxidation takes. Only Cr is here jiska oxidation ho raha hai from +3 oxdn state to +6 oxdn state. So , A is the answer.
Conductivity (unit Siemen’s S) is directly proportional toarea of the vessel and the concentration of the solution in itand is inversely proportional to the length of the vesselthen the unit of the constant of proportionality is [2002]- a)Sm mol–1
- b)Sm2 mol–1
- c)S–2m2 mol
- d)S2m2 mol–2.
Correct answer is option 'B'. Can you explain this answer?
Conductivity (unit Siemen’s S) is directly proportional toarea of the vessel and the concentration of the solution in itand is inversely proportional to the length of the vesselthen the unit of the constant of proportionality is [2002]
a)
Sm mol–1
b)
Sm2 mol–1
c)
S–2m2 mol
d)
S2m2 mol–2.
|
|
Prateek Choudhury answered |
given :
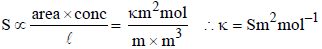
Aluminium oxide may be electrolysed at 1000°C to furnishaluminium metal (At. Mass = 27 amu; 1 Faraday = 96,500Coulombs). The cathode reaction is– Al3+ + 3e- → Al° To prepare 5.12 kg of aluminium metal by this method werequire- a)5.49 × 101 C of electricity
- b)5.49 × 104 C of electricity
- c)1.83 × 107 C of electricity
- d)5.49 × 107 C of electricity
Correct answer is option 'D'. Can you explain this answer?
Aluminium oxide may be electrolysed at 1000°C to furnishaluminium metal (At. Mass = 27 amu; 1 Faraday = 96,500Coulombs). The cathode reaction is– Al3+ + 3e- → Al° To prepare 5.12 kg of aluminium metal by this method werequire
a)
5.49 × 101 C of electricity
b)
5.49 × 104 C of electricity
c)
1.83 × 107 C of electricity
d)
5.49 × 107 C of electricity
|
|
Ishan Chatterjee answered |
1 mole of e– = 1F = 96500 C
27g of Al is deposited by 3 × 96500 C
5120 g of Al will be deposited by
27g of Al is deposited by 3 × 96500 C
5120 g of Al will be deposited by
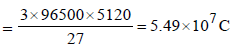
The standard reduction potentials of Cu2+ | Cu and Cu2+|Cu+ are 0.337 V and 0.153 respectively. The standardelectrode potential of Cu+ |Cu half cell is (1997 - 1 Mark)- a)0.184 V
- b)0.827 V
- c)0.521 V
- d)0.490 V
Correct answer is option 'C'. Can you explain this answer?
The standard reduction potentials of Cu2+ | Cu and Cu2+|Cu+ are 0.337 V and 0.153 respectively. The standardelectrode potential of Cu+ |Cu half cell is (1997 - 1 Mark)
a)
0.184 V
b)
0.827 V
c)
0.521 V
d)
0.490 V
|
|
Dipika Chauhan answered |
We have
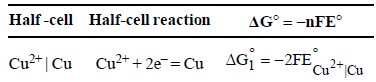
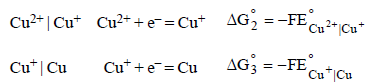
From the half-cell reactions, it follows that
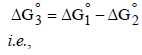
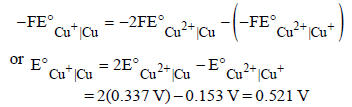
The 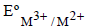 values for Cr, Mn, Fe and Co are – 0.41, + 1.57, + 0.77 and + 1.97V respectively. For which one of these
values for Cr, Mn, Fe and Co are – 0.41, + 1.57, + 0.77 and + 1.97V respectively. For which one of these
metals the change in oxidation state from +2 to +3 is easiest? [2004]- a)Fe
- b)Mn
- c)Cr
- d)Co
Correct answer is option 'C'. Can you explain this answer?
The 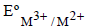 values for Cr, Mn, Fe and Co are – 0.41, + 1.57, + 0.77 and + 1.97V respectively. For which one of these
values for Cr, Mn, Fe and Co are – 0.41, + 1.57, + 0.77 and + 1.97V respectively. For which one of these
metals the change in oxidation state from +2 to +3 is easiest? [2004]
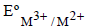 values for Cr, Mn, Fe and Co are – 0.41, + 1.57, + 0.77 and + 1.97V respectively. For which one of these
values for Cr, Mn, Fe and Co are – 0.41, + 1.57, + 0.77 and + 1.97V respectively. For which one of thesemetals the change in oxidation state from +2 to +3 is easiest? [2004]
a)
Fe
b)
Mn
c)
Cr
d)
Co

|
Kirti Choudhary answered |
The given value are reduction potential while M^+2 to +3 is oxidation state so we have to see oxidation potential
The reduction potential of hydrogen half-cell will be negative if :[2011]- a)p(H2) = 1 atm and [H+] = 2.0 M
- b)p(H2) = 1 atm and [H+] = 1.0 M
- c)p(H2) = 2 atm and [H+] = 1.0 M
- d)p(H2) = 2 atm and [H+] = 2.0 M
Correct answer is option 'C'. Can you explain this answer?
The reduction potential of hydrogen half-cell will be negative if :
[2011]
a)
p(H2) = 1 atm and [H+] = 2.0 M
b)
p(H2) = 1 atm and [H+] = 1.0 M
c)
p(H2) = 2 atm and [H+] = 1.0 M
d)
p(H2) = 2 atm and [H+] = 2.0 M
|
|
Rohit Iyer answered |
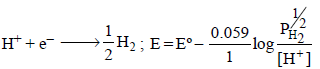
Now if 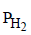 = 2 atm and [H+] = 1M
= 2 atm and [H+] = 1M
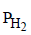 = 2 atm and [H+] = 1M
= 2 atm and [H+] = 1M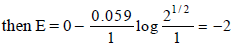
In a galvanic cell, the salt bridge (JEE Adv. 2014)- a)Does not participate chemically in the cell reaction
- b)Stops the diffusion of ions from one electrode toanother
- c)Is necessary for the occurrence of the cell reaction
- d)Ensures mixing of the two electrolytic solutions
Correct answer is option 'A'. Can you explain this answer?
In a galvanic cell, the salt bridge (JEE Adv. 2014)
a)
Does not participate chemically in the cell reaction
b)
Stops the diffusion of ions from one electrode toanother
c)
Is necessary for the occurrence of the cell reaction
d)
Ensures mixing of the two electrolytic solutions
|
|
Gopal Verma answered |
Explanation:
In a galvanic cell, the salt bridge is an essential component that plays a crucial role in the proper functioning of the cell. The correct answer to the question is option 'A', which states that the salt bridge does not participate chemically in the cell reaction. Let's understand why this is the correct answer and explore the role of the salt bridge in a galvanic cell.
Role of the Salt Bridge:
The salt bridge acts as a bridge between the two half-cells of a galvanic cell, allowing the flow of ions and maintaining electrical neutrality. It consists of an electrolyte solution (commonly a salt) that is placed between two half-cells, connecting the anode and cathode compartments. The primary function of the salt bridge is to complete the circuit and facilitate the flow of charge.
Preventing the Build-up of Charge:
When a galvanic cell operates, oxidation occurs at the anode, resulting in the generation of electrons, while reduction occurs at the cathode, consuming electrons. In the absence of a salt bridge, a build-up of charge would occur, preventing further electron flow. This is because the half-cells would become electrically charged due to the accumulation of ions produced during the redox reactions. The salt bridge helps in preventing this build-up of charge and allows continuous electron flow.
Ensuring Electrical Neutrality:
As the oxidation and reduction reactions take place in the two half-cells, ions are produced. These ions need to move to balance the charge and maintain electrical neutrality. The salt bridge allows the migration of these ions from one half-cell to another, ensuring that both half-cells remain electrically neutral. The movement of these ions occurs through the electrolyte solution present in the salt bridge.
Facilitating the Flow of Ions:
In addition to maintaining electrical neutrality, the salt bridge also facilitates the flow of ions between the two half-cells. It allows the exchange of cations and anions, which are necessary for the completion of the redox reactions. This movement of ions helps in maintaining a concentration gradient, allowing the reactions to proceed smoothly.
Conclusion:
In conclusion, the salt bridge in a galvanic cell does not participate chemically in the cell reaction but plays a crucial role in maintaining electrical neutrality, preventing the build-up of charge, and facilitating the flow of ions between the two half-cells. It acts as a bridge, allowing the continuous flow of electrons and completing the circuit.
In a galvanic cell, the salt bridge is an essential component that plays a crucial role in the proper functioning of the cell. The correct answer to the question is option 'A', which states that the salt bridge does not participate chemically in the cell reaction. Let's understand why this is the correct answer and explore the role of the salt bridge in a galvanic cell.
Role of the Salt Bridge:
The salt bridge acts as a bridge between the two half-cells of a galvanic cell, allowing the flow of ions and maintaining electrical neutrality. It consists of an electrolyte solution (commonly a salt) that is placed between two half-cells, connecting the anode and cathode compartments. The primary function of the salt bridge is to complete the circuit and facilitate the flow of charge.
Preventing the Build-up of Charge:
When a galvanic cell operates, oxidation occurs at the anode, resulting in the generation of electrons, while reduction occurs at the cathode, consuming electrons. In the absence of a salt bridge, a build-up of charge would occur, preventing further electron flow. This is because the half-cells would become electrically charged due to the accumulation of ions produced during the redox reactions. The salt bridge helps in preventing this build-up of charge and allows continuous electron flow.
Ensuring Electrical Neutrality:
As the oxidation and reduction reactions take place in the two half-cells, ions are produced. These ions need to move to balance the charge and maintain electrical neutrality. The salt bridge allows the migration of these ions from one half-cell to another, ensuring that both half-cells remain electrically neutral. The movement of these ions occurs through the electrolyte solution present in the salt bridge.
Facilitating the Flow of Ions:
In addition to maintaining electrical neutrality, the salt bridge also facilitates the flow of ions between the two half-cells. It allows the exchange of cations and anions, which are necessary for the completion of the redox reactions. This movement of ions helps in maintaining a concentration gradient, allowing the reactions to proceed smoothly.
Conclusion:
In conclusion, the salt bridge in a galvanic cell does not participate chemically in the cell reaction but plays a crucial role in maintaining electrical neutrality, preventing the build-up of charge, and facilitating the flow of ions between the two half-cells. It acts as a bridge, allowing the continuous flow of electrons and completing the circuit.
For the following electrochemical cell at 298 K, Pt(s)|H2(g, 1 bar)| H+ (aq, 1 M) || M4+ (aq), M2+ (aq)|Pt(s) 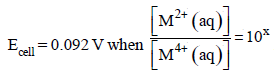 Given :
Given : 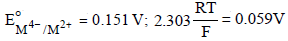 The value of x is (JEE Adv. 2016)
The value of x is (JEE Adv. 2016)- a)–2
- b)–1
- c)1
- d)2
Correct answer is option 'D'. Can you explain this answer?
For the following electrochemical cell at 298 K, Pt(s)|H2(g, 1 bar)| H+ (aq, 1 M) || M4+ (aq), M2+ (aq)|Pt(s)
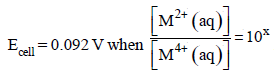
Given : 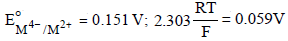
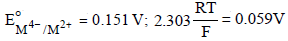
The value of x is (JEE Adv. 2016)
a)
–2
b)
–1
c)
1
d)
2
|
|
Neha Kulkarni answered |
At anode : H2(g) 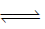 2H+ (aq) + 2e–
2H+ (aq) + 2e–
At cathode : M4+ (aq) + 2e–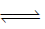 M2+ (aq)
M2+ (aq)
Net cell reaction : H2(g) + M4+ (aq)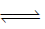 2H+ (aq) + M2+ (aq)
2H+ (aq) + M2+ (aq)
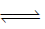 2H+ (aq) + 2e–
2H+ (aq) + 2e–At cathode : M4+ (aq) + 2e–
 M2+ (aq)
M2+ (aq)Net cell reaction : H2(g) + M4+ (aq)
 2H+ (aq) + M2+ (aq)
2H+ (aq) + M2+ (aq)
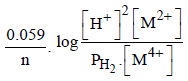
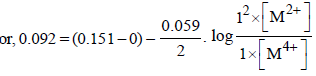
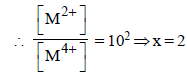
Which of the following chemical reactions depict the oxidizing beahviour of H2SO4?- a)NaCl + H2SO4 → NaHSO4 + HCl
- b)2PCl5 + H2SO4 → 2POCl3 + 2HCl + SO2Cl2
- c)2HI + H2SO4 → I2 + SO2 + 2H2O
- d)Ca(OH)2 + H2SO4 → CaSO4 + 2H2O
Correct answer is option 'C'. Can you explain this answer?
Which of the following chemical reactions depict the oxidizing beahviour of H2SO4?
a)
NaCl + H2SO4 → NaHSO4 + HCl
b)
2PCl5 + H2SO4 → 2POCl3 + 2HCl + SO2Cl2
c)
2HI + H2SO4 → I2 + SO2 + 2H2O
d)
Ca(OH)2 + H2SO4 → CaSO4 + 2H2O
|
|
Pranavi Kumar answered |
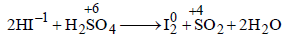 reaction oxidation number of S is decreasing from + 6 to +4 hence undergoing reduction and for HI oxidation
reaction oxidation number of S is decreasing from + 6 to +4 hence undergoing reduction and for HI oxidationNumber of I is increasing from –1 to 0 hence
underegoing oxidation therefore H2SO4 is acting as oxidising agent.
underegoing oxidation therefore H2SO4 is acting as oxidising agent.
For a cell reaction involving a two-electron change, thestandard e.m.f. of the cell is found to be 0.295 V at 25ºC. The equilibrium constant of the reaction at 25ºC will be [2003- a)29.5 × 10–2
- b)10]
- c)1 × 1010
- d)1 × 10–10
Correct answer is option 'C'. Can you explain this answer?
For a cell reaction involving a two-electron change, thestandard e.m.f. of the cell is found to be 0.295 V at 25ºC. The equilibrium constant of the reaction at 25ºC will be [2003
a)
29.5 × 10–2
b)
10]
c)
1 × 1010
d)
1 × 10–10
|
|
Nisha Rane answered |
The equilibrium constant is related to the standard emf of cell by the expression
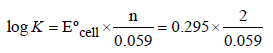
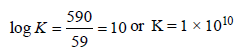
Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox titration. Some half cell reactions and their standard potentials are given below : (2002S)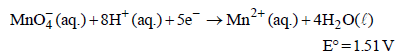
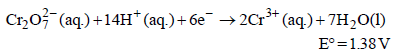 Fe3+ (aq.) + e- → Fe2+ (aq.) E° = 0.77 V
Fe3+ (aq.) + e- → Fe2+ (aq.) E° = 0.77 V
Cl2(g) + 2e- → 2Cl- (aq.) E° = 1.40 VIdentify the only incorrect statement regarding the quantitative estimation of aqueous Fe(NO3)2- a)
 can be used in aqueous HCl
can be used in aqueous HCl - b)
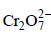 can be used in aqueous HCl
can be used in aqueous HCl - c)
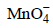 can be used in aqueous H2SO4
can be used in aqueous H2SO4 - d)
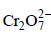 can be used in aqueous H2SO4
can be used in aqueous H2SO4
Correct answer is option 'A'. Can you explain this answer?
Standard electrode potential data are useful for understanding the suitability of an oxidant in a redox titration. Some half cell reactions and their standard potentials are given below : (2002S)
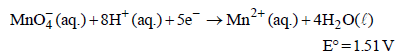
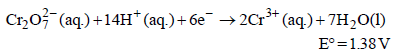
Fe3+ (aq.) + e- → Fe2+ (aq.) E° = 0.77 V
Cl2(g) + 2e- → 2Cl- (aq.) E° = 1.40 V
Cl2(g) + 2e- → 2Cl- (aq.) E° = 1.40 V
Identify the only incorrect statement regarding the quantitative estimation of aqueous Fe(NO3)2
a)
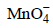 can be used in aqueous HCl
can be used in aqueous HClb)
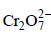 can be used in aqueous HCl
can be used in aqueous HClc)
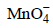 can be used in aqueous H2SO4
can be used in aqueous H2SO4d)
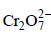 can be used in aqueous H2SO4
can be used in aqueous H2SO4|
|
Rishika Shah answered |
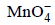 will oxidise Cl– ion according to the following equation
will oxidise Cl– ion according to the following equation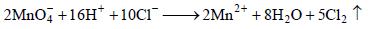
The cell corresponding to this reaction is as follows :
Pt, Cl2 (1 atm) | Cl– || 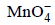 , Mn2+, H+ | Pt
, Mn2+, H+ | Pt
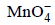 , Mn2+, H+ | Pt
, Mn2+, H+ | Pt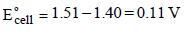
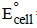 being +ve, ΔG° will be -ve and hence the above reaction is feasible.
being +ve, ΔG° will be -ve and hence the above reaction is feasible. 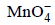 will not only oxidise Fe2+ ion but also Cl– ion simultaneously. So the quantitative estimation of aq Fe(NO3)2 cannot be done by this.
will not only oxidise Fe2+ ion but also Cl– ion simultaneously. So the quantitative estimation of aq Fe(NO3)2 cannot be done by this.Resistance of a conductivity cell filled with a solution of an electrolyte of concentration 0.1 M is 100 W. The conductivity of this solution is 1.29 S m–1. Resistance of the same cell when filled with 0.2 M of the same solution is 520 W. The molar conductivity of 0.2 M solution of electrolyte will be [2006]- a)1.24 × 10–4 S m2 mol–1
- b)12.4 × 10–4 S m2 mol–1
- c)124 × 10–4 S m2 mol–1
- d)1240 × 10–4 S m2 mol–1
Correct answer is option 'B'. Can you explain this answer?
Resistance of a conductivity cell filled with a solution of an electrolyte of concentration 0.1 M is 100 W. The conductivity of this solution is 1.29 S m–1. Resistance of the same cell when filled with 0.2 M of the same solution is 520 W. The molar conductivity of 0.2 M solution of electrolyte will be [2006]
a)
1.24 × 10–4 S m2 mol–1
b)
12.4 × 10–4 S m2 mol–1
c)
124 × 10–4 S m2 mol–1
d)
1240 × 10–4 S m2 mol–1
|
|
Sameer Kumar answered |
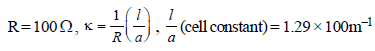
Given, R = 520Ω, C = 0.2 M, μ (molar conductivity) = ?
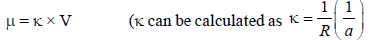
now cell constant is known.)
Hence,
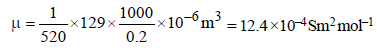
The correct order of  values with negative sign for the four successive elements Cr, Mn, Fe and Co is [2010]
values with negative sign for the four successive elements Cr, Mn, Fe and Co is [2010]- a)Mn > Cr > Fe > Co
- b)Cr < Fe > Mn > Co
- c)Fe > Mn > Cr > Co
- d)Cr > Mn > Fe > Co
Correct answer is option 'A'. Can you explain this answer?
The correct order of 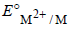 values with negative sign for the four successive elements Cr, Mn, Fe and Co is [2010]
values with negative sign for the four successive elements Cr, Mn, Fe and Co is [2010]
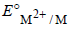 values with negative sign for the four successive elements Cr, Mn, Fe and Co is [2010]
values with negative sign for the four successive elements Cr, Mn, Fe and Co is [2010]a)
Mn > Cr > Fe > Co
b)
Cr < Fe > Mn > Co
c)
Fe > Mn > Cr > Co
d)
Cr > Mn > Fe > Co
|
|
Priya Basu answered |
The value of 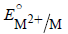 for given metal ions are
for given metal ions are
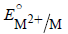 for given metal ions are
for given metal ions are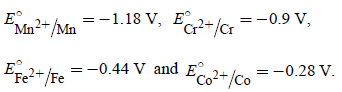
The correct order of 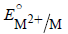 values without
values without
considering negative sign would be
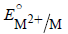 values without
values withoutconsidering negative sign would be
Mn2+ > Cr2+ > Fe2+ > Co2+.
Consider the following Eº values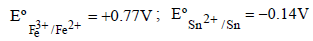 Under standard conditions the potential for the reaction
Under standard conditions the potential for the reaction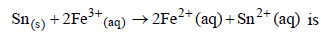 [2004]
[2004]- a)0.91 V
- b)1.40 V
- c)1.68 V
- d)0.63 V
Correct answer is option 'A'. Can you explain this answer?
Consider the following Eº values
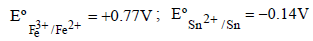
Under standard conditions the potential for the reaction
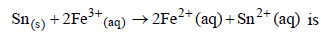 [2004]
[2004]a)
0.91 V
b)
1.40 V
c)
1.68 V
d)
0.63 V
|
|
Anand Kumar answered |
We have given standard reduction potential.
Sn will oxidise at anode and Fe+3 will reduce at cathode
and we know,
Standard reduction potential of cell
= standard reduction of cathode - standard reduction potential of anode
= +0.77v - (-0.14v)
= +0.91v
Sn will oxidise at anode and Fe+3 will reduce at cathode
and we know,
Standard reduction potential of cell
= standard reduction of cathode - standard reduction potential of anode
= +0.77v - (-0.14v)
= +0.91v
A solution of sodium sulphate in water is electrolysed usinginert electrodes. The products at the cathode and anode arerespectively (1987 - 1 Mark)- a)H2, O2
- b)O2, H2
- c)O2, Na
- d)O2, SO2
Correct answer is option 'A'. Can you explain this answer?
A solution of sodium sulphate in water is electrolysed usinginert electrodes. The products at the cathode and anode arerespectively (1987 - 1 Mark)
a)
H2, O2
b)
O2, H2
c)
O2, Na
d)
O2, SO2
|
|
Tanishq Choudhary answered |
Water is reduced at the cathode and oxidized at the anode instead of Na+ and 
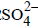
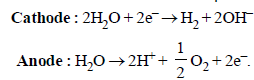
Electrolysis of dilute aqueous NaCl solution was carriedout by passing 10 milli ampere current. The time required toliberate 0.01 mol of H2 gas at the cathode is (1 Faraday =96500 C mol–1) (2008S)- a)9.65 × 104 sec
- b)19.3 × 104 sec
- c)28.95 × 104 sec
- d)38.6 × 104 sec
Correct answer is option 'B'. Can you explain this answer?
Electrolysis of dilute aqueous NaCl solution was carriedout by passing 10 milli ampere current. The time required toliberate 0.01 mol of H2 gas at the cathode is (1 Faraday =96500 C mol–1) (2008S)
a)
9.65 × 104 sec
b)
19.3 × 104 sec
c)
28.95 × 104 sec
d)
38.6 × 104 sec
|
|
Niharika Deshpande answered |
Give : I = 10 milliamperes ; IF = 96500 C mol–1
t = ? ; Moles of H2 produces = 0.01 mol
From the law of electrolysis, we have
Equivalents of H2 produces =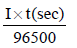
t = ? ; Moles of H2 produces = 0.01 mol
From the law of electrolysis, we have
Equivalents of H2 produces =
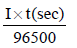
Substituting given values, we get
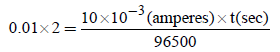
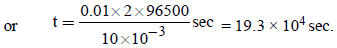
i.e. (b) is the correct answer
What will be the emf for the given cell [2002]
Pt | H2 (P1) | H+ (aq) | | H2 (P2) | Pt- a)
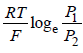
- b)
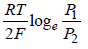
- c)
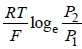
- d)None of these.
Correct answer is option 'B'. Can you explain this answer?
What will be the emf for the given cell [2002]
Pt | H2 (P1) | H+ (aq) | | H2 (P2) | Pt
Pt | H2 (P1) | H+ (aq) | | H2 (P2) | Pt
a)
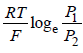
b)
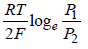
c)
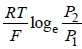
d)
None of these.
|
|
Tanuja Kapoor answered |
Oxidation half call:-
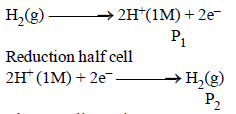
The net cell reaction
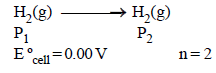
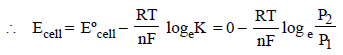
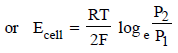
In the electrolytic cell, flow of electrons is from (2003S)- a)Cathode to anode in solution
- b)Cathode to anode through external supply
- c)Cathode to anode through internal supply
- d)Anode to cathode through internal supply
Correct answer is option 'C'. Can you explain this answer?
In the electrolytic cell, flow of electrons is from (2003S)
a)
Cathode to anode in solution
b)
Cathode to anode through external supply
c)
Cathode to anode through internal supply
d)
Anode to cathode through internal supply
|
|
Rahul Bansal answered |
The anode is the electrode at which the oxidation half-reaction takes place. ... In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the electrode with the positive potential.
In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is
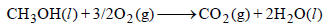
At 298 K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and and CO2 (g) are –166.2, –237.2 and –394.4 kJ
mol–1 respectively. If standard enthalpy of combustion of methonal is – 726 kJ mol–1, efficiency of the fuel cell will be: [2009]- a)87%
- b)90%
- c)97%
- d)80%
Correct answer is option 'C'. Can you explain this answer?
In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is
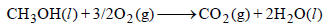
At 298 K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and and CO2 (g) are –166.2, –237.2 and –394.4 kJ
mol–1 respectively. If standard enthalpy of combustion of methonal is – 726 kJ mol–1, efficiency of the fuel cell will be:
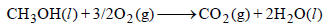
At 298 K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and and CO2 (g) are –166.2, –237.2 and –394.4 kJ
mol–1 respectively. If standard enthalpy of combustion of methonal is – 726 kJ mol–1, efficiency of the fuel cell will be:
[2009]
a)
87%
b)
90%
c)
97%
d)
80%
|
|
Aarav Kumar answered |
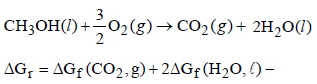
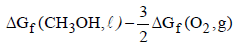
= – 394.4 + 2 (–237.2) – (–166.2) – 0
= – 394.4 – 474.4 + 166.2 = – 702.6 k J
% efficiency =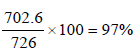
= – 394.4 – 474.4 + 166.2 = – 702.6 k J
% efficiency =
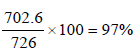
In a hydrogen-oxygen fuel cell, combustion of hydrogenoccurs to [2004]- a)produce high purity water
- b)create potential difference between two electrodes
- c)generate heat
- d)remove adsorbed oxygen from elctrode surfaces
Correct answer is option 'B'. Can you explain this answer?
In a hydrogen-oxygen fuel cell, combustion of hydrogenoccurs to [2004]
a)
produce high purity water
b)
create potential difference between two electrodes
c)
generate heat
d)
remove adsorbed oxygen from elctrode surfaces
|
|
Soumya Nambiar answered |
In H2 -O2 fuel cell, the combustion of H2 occurs to create potential difference between the two electrodes
The reaction :
½ Hg2(g) + AgCl(s) → H+(aq) + Cl–(aq) + Ag(s) occurs in the galvanic cell- a)Ag | AgCl(s) | KCl (soln) | AgNO3 (soln) | Ag
- b)Pt | H2(g) | HCl (soln) | AgNO3 (soln) | Ag
- c)Pt | H2(g) | HCl (soln) | AgCl(s) | Ag
- d)Pt | H2(g) | KCl (soln) | AgCl(s) | Ag
Correct answer is option 'C'. Can you explain this answer?
The reaction :
½ Hg2(g) + AgCl(s) → H+(aq) + Cl–(aq) + Ag(s) occurs in the galvanic cell
½ Hg2(g) + AgCl(s) → H+(aq) + Cl–(aq) + Ag(s) occurs in the galvanic cell
a)
Ag | AgCl(s) | KCl (soln) | AgNO3 (soln) | Ag
b)
Pt | H2(g) | HCl (soln) | AgNO3 (soln) | Ag
c)
Pt | H2(g) | HCl (soln) | AgCl(s) | Ag
d)
Pt | H2(g) | KCl (soln) | AgCl(s) | Ag
|
|
Jatin Kulkarni answered |
NOTE : Oxidation is loss of electron and in a galvanic cell it occurs at anode. Reduction is gain of electron and in a galvanic cell it occurs at cathode.
Cell representation :
Anode / Anodic electrolyte || Cathodic electrolyte / Cathode
Reaction at Anode : H2 → 2H+ + 2e–
Reaction at Cathode : AgCl + e– → Ag + Cl–
Cell representation :
Anode / Anodic electrolyte || Cathodic electrolyte / Cathode
Reaction at Anode : H2 → 2H+ + 2e–
Reaction at Cathode : AgCl + e– → Ag + Cl–
The emf of the cell Zn | Zn2+ (0.01 M) | | Fe2+ (0.001 M) | Fe at 298 K is 0.2905 then the value of equilibrium constant for
the cell reaction is (2004S)- a)
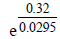
- b)
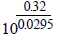
- c)
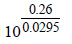
- d)
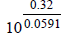
Correct answer is option 'B'. Can you explain this answer?
The emf of the cell Zn | Zn2+ (0.01 M) | | Fe2+ (0.001 M) | Fe at 298 K is 0.2905 then the value of equilibrium constant for
the cell reaction is (2004S)
the cell reaction is (2004S)
a)
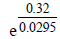
b)
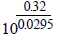
c)
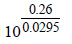
d)
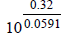
|
|
Jatin Sen answered |
TIPS/FORMULAE :
Use Nernst's equation;
Cell reaction : Zn + Fe2+ → Zn2+ + Fe
Using Nernst equation
Use Nernst's equation;
Cell reaction : Zn + Fe2+ → Zn2+ + Fe
Using Nernst equation
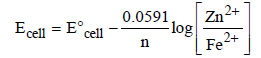
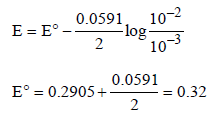
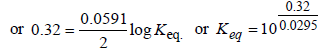
The standard reduction potentials for Zn2+/Zn, Ni2+/Ni and Fe2+/Fe are –0.76,–0.23 and –0.44 V respectively.
The reaction X+Y2+ →X2++Y will be spontaneous when :- a)X = Ni, Y = Fe
- b)X = Ni, Y = Zn
- c)X= Fe, Y = Zn
- d)X= Zn, Y = Ni
Correct answer is option 'D'. Can you explain this answer?
The standard reduction potentials for Zn2+/Zn, Ni2+/Ni and Fe2+/Fe are –0.76,–0.23 and –0.44 V respectively.
The reaction X+Y2+ →X2++Y will be spontaneous when :
The reaction X+Y2+ →X2++Y will be spontaneous when :
a)
X = Ni, Y = Fe
b)
X = Ni, Y = Zn
c)
X= Fe, Y = Zn
d)
X= Zn, Y = Ni
|
|
Aarya Menon answered |
For a spontaneous reaction DG must be –ve
Since ΔG = – nFE°
Hence for ΔG to be -ve ΔE° has to be positive. Which is possible when X = Zn, Y = Ni
Zn + Ni++ → Zn++ + Ni
Since ΔG = – nFE°
Hence for ΔG to be -ve ΔE° has to be positive. Which is possible when X = Zn, Y = Ni
Zn + Ni++ → Zn++ + Ni
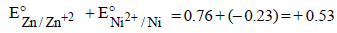
For the electrochemical cell, M|M+ || X- | X,E°M+/M =0.44V and E°(X/X–) = 0.33V.From this data one can deduce that (2000S)- a)M + X → M+ + X- is the spontaneous reaction
- b)M+ + X- →M+ X is the spontaneous reaction
- c)Ecell = 0.77V
- d)Ecell = – 0.77 V
Correct answer is option 'B'. Can you explain this answer?
For the electrochemical cell, M|M+ || X- | X,E°M+/M =0.44V and E°(X/X–) = 0.33V.From this data one can deduce that (2000S)
a)
M + X → M+ + X- is the spontaneous reaction
b)
M+ + X- →M+ X is the spontaneous reaction
c)
Ecell = 0.77V
d)
Ecell = – 0.77 V
|
|
Nishanth Gupta answered |
For M+ + X– → M + X, 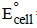 = 0.44 – 0.33 = 0.11V is positive, hence reaction is spontaneous
= 0.44 – 0.33 = 0.11V is positive, hence reaction is spontaneous
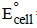 = 0.44 – 0.33 = 0.11V is positive, hence reaction is spontaneous
= 0.44 – 0.33 = 0.11V is positive, hence reaction is spontaneousThe standard reduction potential values of three metalliccations, X, Y and Z are 0.52,– 3.03 and – 1.18 V respectively.The order of reducing power of the corresponding metals is (1998 - 2 Marks)- a)Y > Z > X
- b)X > Y > Z
- c)Z > Y > X
- d)Z > X > Y
Correct answer is option 'A'. Can you explain this answer?
The standard reduction potential values of three metalliccations, X, Y and Z are 0.52,– 3.03 and – 1.18 V respectively.The order of reducing power of the corresponding metals is (1998 - 2 Marks)
a)
Y > Z > X
b)
X > Y > Z
c)
Z > Y > X
d)
Z > X > Y
|
|
Tanuja Kapoor answered |
NOTE : More negative or lower is the reduction potential , more is the reducing property. Thus the reducing power of the corresponding metal will follow the reverse order, i.e. Y > Z > X.
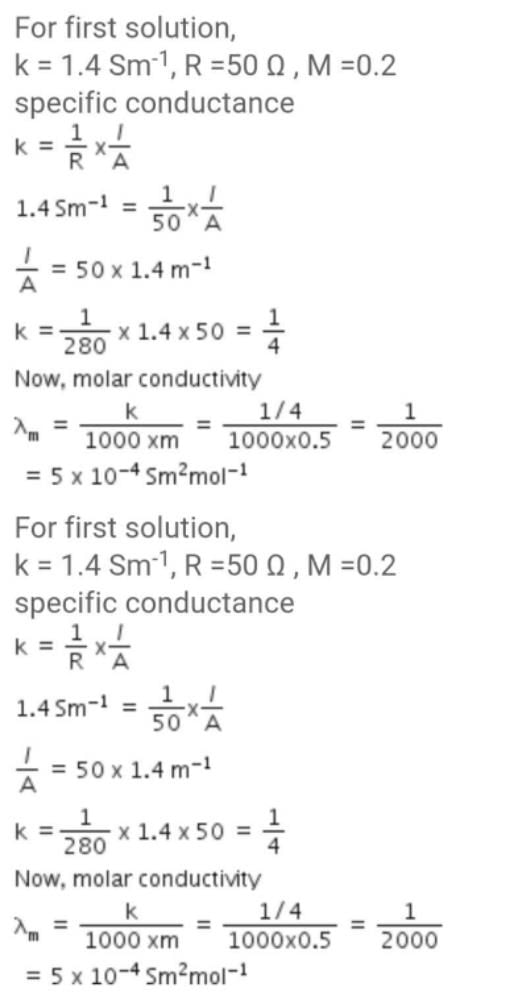
Resistance of 0.2 M solution of an electrolyte is 50 Ω.The specific conductance of the solution is 1.4 S m–1.The resistance of 0.5 M solution of the same electrolyte is 280 Ω. The molar conductivity of 0.5 M solution of the electrolyte in S m2 mol–1 is:[JEE M 2014]- a)5 × 10–4
- b)5 × 10–3
- c)5 × 103
- d)5 × 102
Correct answer is option 'A'. Can you explain this answer?
Resistance of 0.2 M solution of an electrolyte is 50 Ω.The specific conductance of the solution is 1.4 S m–1.The resistance of 0.5 M solution of the same electrolyte is 280 Ω. The molar conductivity of 0.5 M solution of the electrolyte in S m2 mol–1 is:
[JEE M 2014]
a)
5 × 10–4
b)
5 × 10–3
c)
5 × 103
d)
5 × 102
|
|
Neer Shreyansh answered |
The electric charge for electrode deposition of one gramequivalent of a substance is : (1984 - 1 Mark)- a)one ampere per second.
- b)96,500 coloumbs per second.
- c)one ampere for one hour.
- d)charge on one mole of electrons.
Correct answer is option 'D'. Can you explain this answer?
The electric charge for electrode deposition of one gramequivalent of a substance is : (1984 - 1 Mark)
a)
one ampere per second.
b)
96,500 coloumbs per second.
c)
one ampere for one hour.
d)
charge on one mole of electrons.
|
|
Rajeev Mehra answered |
Charge of one mole of electrons = 96500 C ∴ 1 mole gram equivalent of substance will be deposited by one mole of electrons.
A dilute aqueous solution of Na2SO4 is electrolyzed using platinum electrodes.The products at the anode and cathode
are:- a)O2, H2
- b)
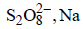
- c)O2 ,Na
- d)
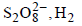
Correct answer is option 'A'. Can you explain this answer?
A dilute aqueous solution of Na2SO4 is electrolyzed using platinum electrodes.The products at the anode and cathode
are:
are:
a)
O2, H2
b)
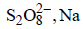
c)
O2 ,Na
d)
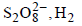
|
|
Maulik Majumdar answered |
H2O is more readily reduced at cathode than Na+. It is also more readily oxidized at anode than 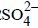 . Hence, the electrode reactions are
. Hence, the electrode reactions are
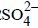 . Hence, the electrode reactions are
. Hence, the electrode reactions are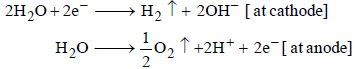
The cell, Zn | Zn2+ (1 M) || Cu2+ (1 M) | Cu 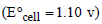 was allowed to be completely discharged at 298 K. The relative
was allowed to be completely discharged at 298 K. The relative
concentration of 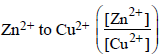 is [2007]
is [2007] - a)9.65 × 104
- b)antilog (24.08)
- c)37.3
- d)1037.3
Correct answer is option 'D'. Can you explain this answer?
The cell, Zn | Zn2+ (1 M) || Cu2+ (1 M) | Cu 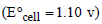 was allowed to be completely discharged at 298 K. The relative
was allowed to be completely discharged at 298 K. The relative
concentration of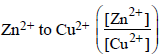 is [2007]
is [2007]
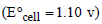 was allowed to be completely discharged at 298 K. The relative
was allowed to be completely discharged at 298 K. The relativeconcentration of
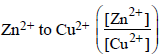 is [2007]
is [2007] a)
9.65 × 104
b)
antilog (24.08)
c)
37.3
d)
1037.3
|
|
Ashwin Verma answered |
Ecell = 0; when cell is completely discharged.
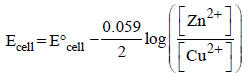
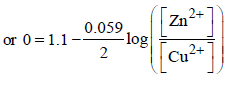
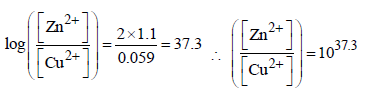
Chapter doubts & questions for Electrochemistry - 35 Years Chapter wise Previous Year Solved Papers for JEE 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Electrochemistry - 35 Years Chapter wise Previous Year Solved Papers for JEE in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily

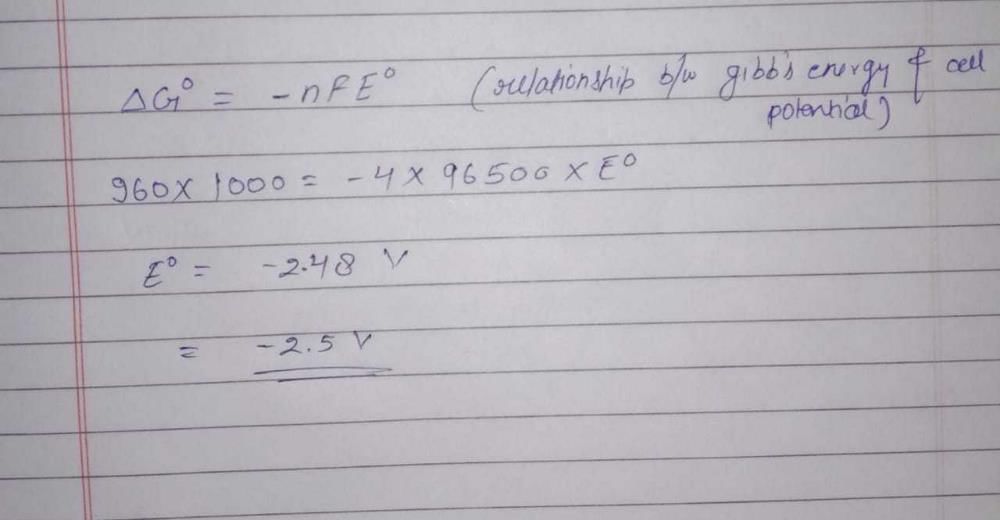
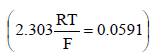 [2003]
[2003]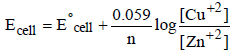
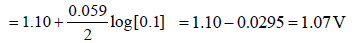
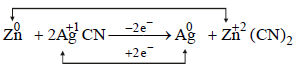
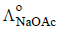 and
and 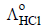 at infinite dilution in water at 25ºC are 91.0 and 426.2 S cm2/mol
at infinite dilution in water at 25ºC are 91.0 and 426.2 S cm2/mol , the additional value required is
, the additional value required is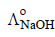
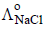
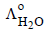
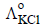

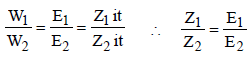


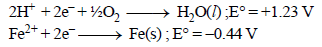
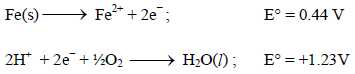
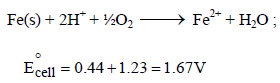
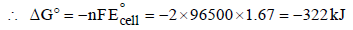
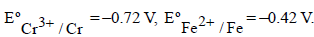 The potential for the cell Cr|Cr3+ (0.1M)|| Fe2+ (0.01 M)| Fe is [2008]
The potential for the cell Cr|Cr3+ (0.1M)|| Fe2+ (0.01 M)| Fe is [2008]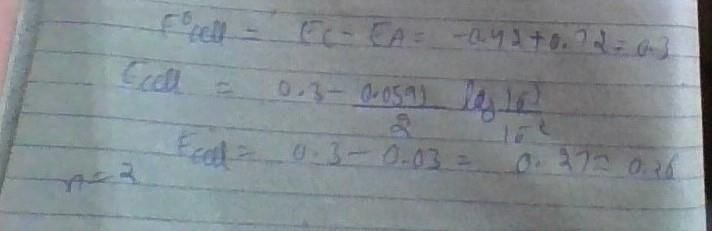
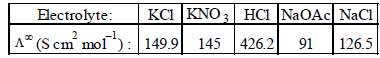
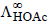 using appropriate molar conductances of the electrolytes listed above at infinite dilution in H2O
using appropriate molar conductances of the electrolytes listed above at infinite dilution in H2O