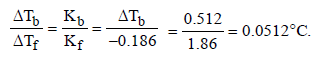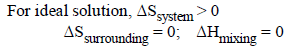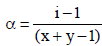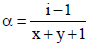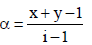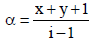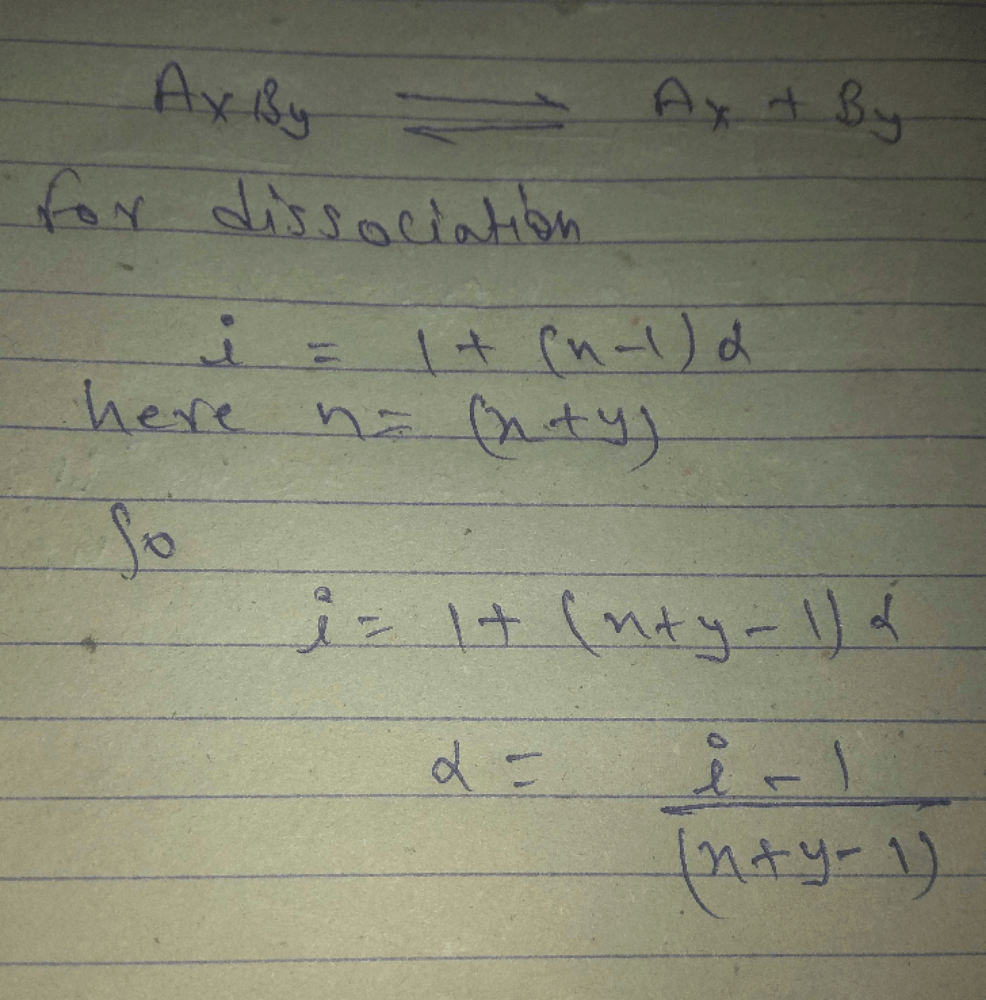All Exams >
JEE >
35 Years Chapter wise Previous Year Solved Papers for JEE >
All Questions
All questions of Solutions for JEE Exam
When 20 g of naphthoic acid (C11H8O2) is dissolved in 50g of benzene (Kf = 1.72 K kg mol–1), a freezing pointdepression of 2K is observed. The Van't Hoff factor (i) is (2007)- a)0.5
- b)1
- c)2
- d)3
Correct answer is option 'A'. Can you explain this answer?
When 20 g of naphthoic acid (C11H8O2) is dissolved in 50g of benzene (Kf = 1.72 K kg mol–1), a freezing pointdepression of 2K is observed. The Van't Hoff factor (i) is (2007)
a)
0.5
b)
1
c)
2
d)
3
|
|
Chirag Verma answered |
Molecular weight of naphthoic acid
C11H8O2 = 172 gmol–1.
The theoretical value of depression in freezing point
C11H8O2 = 172 gmol–1.
The theoretical value of depression in freezing point
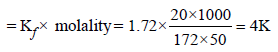
Van't Hoff factor,
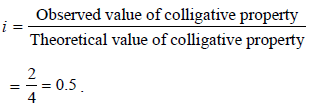
For a dilute solution, Raoult’s law states that :(1985 - 1 Mark)- a)the lowering of vapour pressure is equal to the molefraction of solute.
- b)the relative lowering of vapour pressure is equal to themole fraction of solute.
- c)the relative lowering of vapour pressure is proportionalto the amount of solute in solution.
- d)the vapour pressure of the solution is equal to themole fraction of solvent.
Correct answer is option 'B'. Can you explain this answer?
For a dilute solution, Raoult’s law states that :(1985 - 1 Mark)
a)
the lowering of vapour pressure is equal to the molefraction of solute.
b)
the relative lowering of vapour pressure is equal to themole fraction of solute.
c)
the relative lowering of vapour pressure is proportionalto the amount of solute in solution.
d)
the vapour pressure of the solution is equal to themole fraction of solvent.

|
Sanchita Iyer answered |
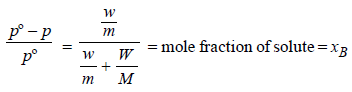
[Mathematical statement of Raoult's law]
The freezing point (in °C) of a solution containing 0.1 gof K3[Fe(CN)6] (Mol. wt. 329) in 100 g of water (Kf = 1.86 Kkg mol–1) is (2011)- a)–2.3 × 10–2
- b)–5.7 × 10–2
- c)–5.7 × 10–3
- d)–1.2 × 10–2
Correct answer is option 'A'. Can you explain this answer?
The freezing point (in °C) of a solution containing 0.1 gof K3[Fe(CN)6] (Mol. wt. 329) in 100 g of water (Kf = 1.86 Kkg mol–1) is (2011)
a)
–2.3 × 10–2
b)
–5.7 × 10–2
c)
–5.7 × 10–3
d)
–1.2 × 10–2
|
|
Lavanya Menon answered |
ΔTf = i × Kf × m
Where m = Molality of the solution
(i.e. number of moles of solute per 1000 g of the solvent)
Where m = Molality of the solution
(i.e. number of moles of solute per 1000 g of the solvent)
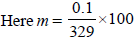
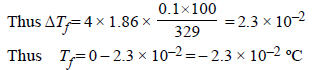
Benzene and toluene form nearly ideal solution. At 20°C,the vapour pressure of benzene is 75 torr and that of tolueneis 22 torr. The partial vapour pressure of benzene at 20°C fora solution containing 78 g of benzene and 46 g of toluene intorr is [2005]- a)53.5
- b)37.5
- c)25
- d)50
Correct answer is option 'D'. Can you explain this answer?
Benzene and toluene form nearly ideal solution. At 20°C,the vapour pressure of benzene is 75 torr and that of tolueneis 22 torr. The partial vapour pressure of benzene at 20°C fora solution containing 78 g of benzene and 46 g of toluene intorr is [2005]
a)
53.5
b)
37.5
c)
25
d)
50
|
|
Akshara Nair answered |
°C, the vapor pressure of pure benzene is 74 torr and the vapor pressure of pure toluene is 22 torr. When a solution containing 50.0 g of benzene and 50.0 g of toluene is prepared, the vapor pressure of the solution is 38 torr. Calculate the mole fraction of benzene in the solution.
To solve this problem, we can use Raoult's Law, which states that the partial pressure of a component in a solution is equal to the product of its mole fraction and its vapor pressure in its pure state.
Let's denote the mole fraction of benzene as x and the mole fraction of toluene as (1-x).
According to Raoult's Law, the partial pressure of benzene (Pbenzene) in the solution is given by:
Pbenzene = x * Pbenzene(pure)
Similarly, the partial pressure of toluene (Ptoluene) in the solution is given by:
Ptoluene = (1-x) * Ptoluene(pure)
Given that Pbenzene(pure) = 74 torr, Ptoluene(pure) = 22 torr, and the total vapor pressure of the solution (Ptotal) = 38 torr, we can set up the following equation:
Pbenzene + Ptoluene = Ptotal
Substituting the expressions for Pbenzene and Ptoluene:
x * Pbenzene(pure) + (1-x) * Ptoluene(pure) = Ptotal
x * 74 + (1-x) * 22 = 38
Simplifying the equation:
74x + 22 - 22x = 38
52x = 16
x = 16/52
x = 0.3077
Therefore, the mole fraction of benzene in the solution is approximately 0.3077.
To solve this problem, we can use Raoult's Law, which states that the partial pressure of a component in a solution is equal to the product of its mole fraction and its vapor pressure in its pure state.
Let's denote the mole fraction of benzene as x and the mole fraction of toluene as (1-x).
According to Raoult's Law, the partial pressure of benzene (Pbenzene) in the solution is given by:
Pbenzene = x * Pbenzene(pure)
Similarly, the partial pressure of toluene (Ptoluene) in the solution is given by:
Ptoluene = (1-x) * Ptoluene(pure)
Given that Pbenzene(pure) = 74 torr, Ptoluene(pure) = 22 torr, and the total vapor pressure of the solution (Ptotal) = 38 torr, we can set up the following equation:
Pbenzene + Ptoluene = Ptotal
Substituting the expressions for Pbenzene and Ptoluene:
x * Pbenzene(pure) + (1-x) * Ptoluene(pure) = Ptotal
x * 74 + (1-x) * 22 = 38
Simplifying the equation:
74x + 22 - 22x = 38
52x = 16
x = 16/52
x = 0.3077
Therefore, the mole fraction of benzene in the solution is approximately 0.3077.
Among the following mixtures, dipole-dipole as the majorinteraction, is present in [2006]- a)KCl and water
- b)benzene and carbon tetrachloride
- c)benzene and ethanol
- d)acetonitrile and acetone
Correct answer is option 'D'. Can you explain this answer?
Among the following mixtures, dipole-dipole as the majorinteraction, is present in [2006]
a)
KCl and water
b)
benzene and carbon tetrachloride
c)
benzene and ethanol
d)
acetonitrile and acetone
|
|
Preeti Iyer answered |
Both acetone and acetonitrile contain a polar bond. Both molecules have what is called a Hydrogen Bond Acceptor, but neither of them have a Hydrogen Bond Donor.
So these two molecules will interact with each other through their polar regions (dipole regions - forming a Dipole-Dipole interaction).
Acetone's polar bond: C=O
Acetonitrile's polar bond: C=N (except it is a triple bond - I don't know how to make a triple bond on here).
The vapour pressure of acetone at 20°C is 185 torr. When1.2 g of a non-volatile substance was dissolved in 100 g ofacetone at 20°C, its vapour pressure was 183 torr. The molarmass (g mol–1) of the substance is : [JEE M 2015]- a)128
- b)488
- c)32
- d)64
Correct answer is option 'D'. Can you explain this answer?
The vapour pressure of acetone at 20°C is 185 torr. When1.2 g of a non-volatile substance was dissolved in 100 g ofacetone at 20°C, its vapour pressure was 183 torr. The molarmass (g mol–1) of the substance is : [JEE M 2015]
a)
128
b)
488
c)
32
d)
64

|
Saxena Aastha answered |
Kyaa chul mach rahi hai bhai
In mixture A and B components show -ve deviation as [2002]- a)Δ Vmix > 0
- b)ΔHmix < 0
- c)A – B interaction is weaker than A – A and B – Binteraction
- d)A – B interaction is stronger than A – A and B – Binteraction.
Correct answer is option 'D'. Can you explain this answer?
In mixture A and B components show -ve deviation as [2002]
a)
Δ Vmix > 0
b)
ΔHmix < 0
c)
A – B interaction is weaker than A – A and B – Binteraction
d)
A – B interaction is stronger than A – A and B – Binteraction.
|
|
Anisha Sengupta answered |
In solution containing A and B component showing negative deviation A–A and B–B interactions are weaker than that of A–B interactions. For such solutions.
ΔH = –ve and ΔV = –ve
ΔH = –ve and ΔV = –ve
Equimolar solutions in the same solvent have [2005]- a)Different boiling and different freezing points
- b)Same boiling and same freezing points
- c)Same freezing point but different boiling points
- d)Same boiling point but different freezing points
Correct answer is option 'D'. Can you explain this answer?
Equimolar solutions in the same solvent have [2005]
a)
Different boiling and different freezing points
b)
Same boiling and same freezing points
c)
Same freezing point but different boiling points
d)
Same boiling point but different freezing points
|
|
Gaurav Choudhury answered |
Equimolar solutions in the same solvent have the same number of particles per unit volume, which means that they will exhibit similar colligative properties. Colligative properties depend only on the number of solute particles in the solution and not on their chemical nature. Therefore, equimolar solutions in the same solvent will have the same boiling point elevation and freezing point depression. However, the magnitude of these effects will depend on the concentration of the solute in the solution.
Same boiling point but different freezing points
Equimolar solutions in the same solvent will have the same boiling point elevation because the vapor pressure of the solution will be lowered by an equal amount due to the presence of the solute particles. This means that the boiling point will be raised by the same amount for both solutions. However, the freezing point depression will depend on the concentration of the solute in the solution. Therefore, equimolar solutions in the same solvent may have different freezing points.
Example
For example, consider equimolar solutions of glucose and urea in water. Both solutions will have the same boiling point elevation because they have the same number of solute particles per unit volume. However, the freezing point depression will be greater for the glucose solution because glucose is a larger molecule than urea and will therefore interact more strongly with the water molecules, lowering the freezing point by a greater amount.
Conclusion
Therefore, the correct option is 'D' - equimolar solutions in the same solvent have the same boiling point but different freezing points.
Same boiling point but different freezing points
Equimolar solutions in the same solvent will have the same boiling point elevation because the vapor pressure of the solution will be lowered by an equal amount due to the presence of the solute particles. This means that the boiling point will be raised by the same amount for both solutions. However, the freezing point depression will depend on the concentration of the solute in the solution. Therefore, equimolar solutions in the same solvent may have different freezing points.
Example
For example, consider equimolar solutions of glucose and urea in water. Both solutions will have the same boiling point elevation because they have the same number of solute particles per unit volume. However, the freezing point depression will be greater for the glucose solution because glucose is a larger molecule than urea and will therefore interact more strongly with the water molecules, lowering the freezing point by a greater amount.
Conclusion
Therefore, the correct option is 'D' - equimolar solutions in the same solvent have the same boiling point but different freezing points.
0.2 molal acid HX is 20% ionised in solution. Kf = 1.86 Kmolality–1. The freezing point of the solution is : (1995S)- a)– 0.45
- b)– 0.90
- c)– 0.31
- d)– 0.53
Correct answer is option 'A'. Can you explain this answer?
0.2 molal acid HX is 20% ionised in solution. Kf = 1.86 Kmolality–1. The freezing point of the solution is : (1995S)
a)
– 0.45
b)
– 0.90
c)
– 0.31
d)
– 0.53
|
|
Manasa Kaur answered |
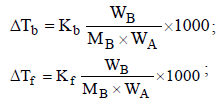
Depression in freezing point, 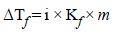
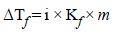
Van’t Hoff factor, 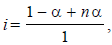 where n = no. of ions
where n = no. of ions
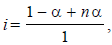 where n = no. of ions
where n = no. of ions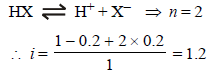
[ For 20 % ionisation, α = 20/100= 0.2]
∴ΔTf = 1.2 × 1.86 × 0.2 = 0.45 [ ∵ m = 0.2]
Hence freezing point of solution is 0 – 0.45 = –0.45
[∵ F.P of water = 0.0 C]
∴ΔTf = 1.2 × 1.86 × 0.2 = 0.45 [ ∵ m = 0.2]
Hence freezing point of solution is 0 – 0.45 = –0.45
[∵ F.P of water = 0.0 C]
The elevation in boiling point of a solution of 13.44 g ofCuCl2 in 1 kg of water using the following information will be(Molecular weight of CuCl2 = 134.4 and Kb = 0.52 K molal-1) (2005S)- a)0.16
- b)0.05
- c)0.1
- d)0.2
Correct answer is option 'A'. Can you explain this answer?
The elevation in boiling point of a solution of 13.44 g ofCuCl2 in 1 kg of water using the following information will be(Molecular weight of CuCl2 = 134.4 and Kb = 0.52 K molal-1) (2005S)
a)
0.16
b)
0.05
c)
0.1
d)
0.2
|
|
Mansi Sarkar answered |
TIPS/Formulae :

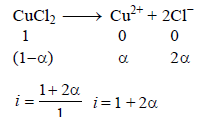
Assuming 100% ionization So, i = 1 + 2 = 3
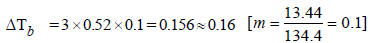
If liquids A and B form an ideal solution [2003]- a)the entropy of mixing is zero
- b)the free energy of mixing is zero
- c)the free energy as well as the entropy of mixing areeach zero
- d)the enthalpy of mixing is zero
Correct answer is option 'D'. Can you explain this answer?
If liquids A and B form an ideal solution [2003]
a)
the entropy of mixing is zero
b)
the free energy of mixing is zero
c)
the free energy as well as the entropy of mixing areeach zero
d)
the enthalpy of mixing is zero
|
|
Vivek answered |
Bcoz there is no difference in the type of bonding or interaction between the two liquids and within the liquids themselves , no heat will be released.
A 5.2 molal aqueous solution of methyl alcohol, CH3OH, issupplied. What is the mole fraction of methyl alcohol in thesolution? [2011]- a)0.100
- b)0.190
- c)0.086
- d)0.050
Correct answer is option 'C'. Can you explain this answer?
A 5.2 molal aqueous solution of methyl alcohol, CH3OH, issupplied. What is the mole fraction of methyl alcohol in thesolution? [2011]
a)
0.100
b)
0.190
c)
0.086
d)
0.050

|
Ashok Khamkar answered |
5.2 molal solution means 5.2 moles of methylalcohal in 1000 gm water
mole solution of methylalcohal
= moles of methylalcohal /moles of methylalcohal +moles of water
= 5.2/5.2+1000/18
= 0.086
mole solution of methylalcohal
= moles of methylalcohal /moles of methylalcohal +moles of water
= 5.2/5.2+1000/18
= 0.086
A binary liquid solution is prepared by mixing n-heptaneand ethanol. Which one of the following statements iscorrect regarding the behaviour of the solution? [2009]- a)The solution is non-ideal, showing – ve deviation fromRaoult’s Law.
- b)The solution is non-ideal, showing + ve deviation fromRaoult’s Law.
- c)n-heptane shows + ve deviation while ethanol shows– ve deviation from Raoult’s Law.
- d)The solution formed is an ideal solution.
Correct answer is option 'B'. Can you explain this answer?
A binary liquid solution is prepared by mixing n-heptaneand ethanol. Which one of the following statements iscorrect regarding the behaviour of the solution? [2009]
a)
The solution is non-ideal, showing – ve deviation fromRaoult’s Law.
b)
The solution is non-ideal, showing + ve deviation fromRaoult’s Law.
c)
n-heptane shows + ve deviation while ethanol shows– ve deviation from Raoult’s Law.
d)
The solution formed is an ideal solution.
|
|
Anjana Sharma answered |
(B) : The solution containing n-heptane and ethanol shows non-ideal behaviour with positive deviation from Raoult’s law. This is because the ethanol molecules are held together by strong H-bonds, however the forces between n-heptane and ethanol are not very strong, as a result they easily vapourise showing higher vapour presure than expected.
During depression of freezing point in a solution thefollowing are in equililbrium (2003S)- a)liquid solvent, solid solvent
- b)liquid solvent, solid solute
- c)liquid solute, solid solute
- d)liquid solute, solid solvent
Correct answer is option 'A'. Can you explain this answer?
During depression of freezing point in a solution thefollowing are in equililbrium (2003S)
a)
liquid solvent, solid solvent
b)
liquid solvent, solid solute
c)
liquid solute, solid solute
d)
liquid solute, solid solvent
|
|
Nilanjan Mehra answered |
NOTE : At the freezing point liquid and solid remain in equilibrium. If a solution of a non-volatile solute is cooled to a temperature below the freezing point of solution, some of liquid solvent will separate as a solid solvent and thus the concentration of solution will
increase.
increase.
If sodium sulphate is considered to be completelydissociated into cations and anions in aqueous solution,the change in freezing point of water (ΔTf), when 0.01 molof sodium sulphate is dissolved in 1 kg of water, is(Kf = 1.86 K kg mol–1) [2010]- a)0.372 K
- b)0.0558 K
- c)0.0744 K
- d)0.0186 K
Correct answer is option 'B'. Can you explain this answer?
If sodium sulphate is considered to be completelydissociated into cations and anions in aqueous solution,the change in freezing point of water (ΔTf), when 0.01 molof sodium sulphate is dissolved in 1 kg of water, is(Kf = 1.86 K kg mol–1) [2010]
a)
0.372 K
b)
0.0558 K
c)
0.0744 K
d)
0.0186 K
|
|
Madhurima Patel answered |
ΔTf) can be calculated using the equation:
ΔTf = Kf * molality
where Kf is the freezing point depression constant of water (1.86 °C/m) and molality is the molal concentration of the solute (in moles of solute per kilogram of solvent).
For sodium sulphate (Na2SO4), the molal concentration can be calculated as follows:
Molar mass of Na2SO4 = 142.04 g/mol
Number of moles of Na2SO4 = mass of Na2SO4 / molar mass of Na2SO4
Assuming 1 kg of water, mass of Na2SO4 required to make a 1 molal solution = 1 mol * 142.04 g/mol = 142.04 g
Molality of Na2SO4 solution = number of moles of Na2SO4 / mass of water in kg = (142.04 g / 142.04 g/mol) / 1 kg = 1 mol/kg
Substituting these values into the equation for ΔTf, we get:
ΔTf = 1.86 * 1 = 1.86 °C
Therefore, the change in freezing point of water due to the addition of sodium sulphate is 1.86 °C.
ΔTf = Kf * molality
where Kf is the freezing point depression constant of water (1.86 °C/m) and molality is the molal concentration of the solute (in moles of solute per kilogram of solvent).
For sodium sulphate (Na2SO4), the molal concentration can be calculated as follows:
Molar mass of Na2SO4 = 142.04 g/mol
Number of moles of Na2SO4 = mass of Na2SO4 / molar mass of Na2SO4
Assuming 1 kg of water, mass of Na2SO4 required to make a 1 molal solution = 1 mol * 142.04 g/mol = 142.04 g
Molality of Na2SO4 solution = number of moles of Na2SO4 / mass of water in kg = (142.04 g / 142.04 g/mol) / 1 kg = 1 mol/kg
Substituting these values into the equation for ΔTf, we get:
ΔTf = 1.86 * 1 = 1.86 °C
Therefore, the change in freezing point of water due to the addition of sodium sulphate is 1.86 °C.
Kf for water is 1.86 K kg mol–1. If your automobile radiatorholds 1.0 kg of water, how many grams of ethylene glycol(C2H6O2) must you add to get the freezing point of thesolution lowered to –2.8ºC ? [2012]- a)72 g
- b)93 g
- c)39 g
- d)27 g
Correct answer is option 'B'. Can you explain this answer?
Kf for water is 1.86 K kg mol–1. If your automobile radiatorholds 1.0 kg of water, how many grams of ethylene glycol(C2H6O2) must you add to get the freezing point of thesolution lowered to –2.8ºC ? [2012]
a)
72 g
b)
93 g
c)
39 g
d)
27 g
|
|
Parth Majumdar answered |
Understanding the Problem
To find out how much ethylene glycol (C2H6O2) is needed to lower the freezing point of water in the radiator, we can use the formula for freezing point depression:
ΔTf = Kf * m
Where:
- ΔTf = change in freezing point
- Kf = freezing point depression constant for water (1.86 K kg mol–1)
- m = molality of the solution (moles of solute per kg of solvent)
Calculating the Change in Freezing Point
- The normal freezing point of water is 0°C.
- The desired freezing point is -2.8°C.
ΔTf = 0 - (-2.8) = 2.8°C
Finding the Molality
Rearranging the formula gives us:
m = ΔTf / Kf
Substituting the values:
m = 2.8°C / 1.86 K kg mol–1 ≈ 1.505 mol/kg
Since we have 1.0 kg of water in the radiator, the number of moles of ethylene glycol needed is:
Moles of C2H6O2 = molality * kg of solvent
Moles = 1.505 mol/kg * 1.0 kg ≈ 1.505 moles
Calculating the Mass of Ethylene Glycol
The molar mass of ethylene glycol (C2H6O2) is:
- C: 2 * 12.01 g/mol
- H: 6 * 1.008 g/mol
- O: 2 * 16.00 g/mol
Molar mass ≈ 62.07 g/mol
Now, to find the mass:
Mass = moles * molar mass
Mass = 1.505 moles * 62.07 g/mol ≈ 93.34 g
Thus, the amount of ethylene glycol needed is approximately 93 g.
Conclusion
Therefore, to lower the freezing point to -2.8°C, you must add approximately 93 grams of ethylene glycol, confirming that option 'B' is correct.
To find out how much ethylene glycol (C2H6O2) is needed to lower the freezing point of water in the radiator, we can use the formula for freezing point depression:
ΔTf = Kf * m
Where:
- ΔTf = change in freezing point
- Kf = freezing point depression constant for water (1.86 K kg mol–1)
- m = molality of the solution (moles of solute per kg of solvent)
Calculating the Change in Freezing Point
- The normal freezing point of water is 0°C.
- The desired freezing point is -2.8°C.
ΔTf = 0 - (-2.8) = 2.8°C
Finding the Molality
Rearranging the formula gives us:
m = ΔTf / Kf
Substituting the values:
m = 2.8°C / 1.86 K kg mol–1 ≈ 1.505 mol/kg
Since we have 1.0 kg of water in the radiator, the number of moles of ethylene glycol needed is:
Moles of C2H6O2 = molality * kg of solvent
Moles = 1.505 mol/kg * 1.0 kg ≈ 1.505 moles
Calculating the Mass of Ethylene Glycol
The molar mass of ethylene glycol (C2H6O2) is:
- C: 2 * 12.01 g/mol
- H: 6 * 1.008 g/mol
- O: 2 * 16.00 g/mol
Molar mass ≈ 62.07 g/mol
Now, to find the mass:
Mass = moles * molar mass
Mass = 1.505 moles * 62.07 g/mol ≈ 93.34 g
Thus, the amount of ethylene glycol needed is approximately 93 g.
Conclusion
Therefore, to lower the freezing point to -2.8°C, you must add approximately 93 grams of ethylene glycol, confirming that option 'B' is correct.
An azeotropic solution of two liquids has boiling point lowerthan either of them when it (1981 - 1 Mark)- a)shows negative deviation from Raoult’s law
- b)shows no deviation from Raoult’s law
- c)shows positive deviation from Raoult’s law
- d)is saturated
Correct answer is option 'C'. Can you explain this answer?
An azeotropic solution of two liquids has boiling point lowerthan either of them when it (1981 - 1 Mark)
a)
shows negative deviation from Raoult’s law
b)
shows no deviation from Raoult’s law
c)
shows positive deviation from Raoult’s law
d)
is saturated

|
Bhavana Das answered |
Lower the B. pt., higher will be the V.P. The V.P. of the mixture is greater than either of the two liquids.
[NOTE : : In case of positive deviation from Roult's law the partial vapour pressure of each liquid and total vapour pressure of solution will be greater as compared to initial solution]
[NOTE : : In case of positive deviation from Roult's law the partial vapour pressure of each liquid and total vapour pressure of solution will be greater as compared to initial solution]
A pressure cooker reduces cooking time for food because[2003]- a)boiling point of water involved in cooking is increased
- b)the higher pressure inside the cooker crushes the foodmaterial
- c)cooking involves chemical changes helped by a rise intemperature
- d)heat is more evenly distributed in the cooking space
Correct answer is option 'A'. Can you explain this answer?
A pressure cooker reduces cooking time for food because[2003]
a)
boiling point of water involved in cooking is increased
b)
the higher pressure inside the cooker crushes the foodmaterial
c)
cooking involves chemical changes helped by a rise intemperature
d)
heat is more evenly distributed in the cooking space
|
|
Nisha answered |
Due to high pressure inside the boiling point is raised.
On mixing, heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components(heptane and octane) are 105 kPa and 45 kPa respectively.Vapour pressure of the solution obtained by mixing 25.0 gof heptane and 35 g of octane will be(molar mass of heptane = 100 g mol–1 and of octane = 114 gmol–1) [2010]- a)72.0 kPa
- b)36.1 kPa
- c)96.2 kPa
- d)144.5 kPa
Correct answer is option 'A'. Can you explain this answer?
On mixing, heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components(heptane and octane) are 105 kPa and 45 kPa respectively.Vapour pressure of the solution obtained by mixing 25.0 gof heptane and 35 g of octane will be(molar mass of heptane = 100 g mol–1 and of octane = 114 gmol–1) [2010]
a)
72.0 kPa
b)
36.1 kPa
c)
96.2 kPa
d)
144.5 kPa
|
|
Visvesh Subramani answered |
Using Raoult's law:-Total vapour pressure=(partial vapour pressure of heptane + partial vapour pressure of octane)= P1X1 + P2X2Where X is mole fraction of component
100 g heptane = 1 mole. 25 g heptane = 0.25 moles. 114 g octane = 1 mole35 g octane = 35/114 = 0.307 moles
Mole fraction of heptane = n1/(n1 + n2)=0.25/(0.25+0.307)=0.4488
Mole fraction of octane = n2/(n1 + n2)=0.307/(0.25+0.307)=0.5511
Applying this in Raoult's law105(0.4488)+45(0.5511)You get 72 kPa
100 g heptane = 1 mole. 25 g heptane = 0.25 moles. 114 g octane = 1 mole35 g octane = 35/114 = 0.307 moles
Mole fraction of heptane = n1/(n1 + n2)=0.25/(0.25+0.307)=0.4488
Mole fraction of octane = n2/(n1 + n2)=0.307/(0.25+0.307)=0.5511
Applying this in Raoult's law105(0.4488)+45(0.5511)You get 72 kPa
Mixture(s) showing positive deviation from Raoult’s law at35 °C is (are) (JEE Adv. 2016)- a)carbon tetrachloride + methanol
- b)carbon disulphide + acetone
- c)benzene + toluene
- d)phenol + aniline
Correct answer is option 'A,B'. Can you explain this answer?
Mixture(s) showing positive deviation from Raoult’s law at35 °C is (are) (JEE Adv. 2016)
a)
carbon tetrachloride + methanol
b)
carbon disulphide + acetone
c)
benzene + toluene
d)
phenol + aniline

|
Anand Khanna answered |
(A) H-bonding of methanol breaks when CCl4 is added so bonds become weaker, resulting positive deviation.
(B) Mixing of polar and non-polar liquids will produce a solution of weaker interaction, resulting positive deviation
(C) Ideal solution
(D) –ve deviation because stronger H-bond is formed.
(B) Mixing of polar and non-polar liquids will produce a solution of weaker interaction, resulting positive deviation
(C) Ideal solution
(D) –ve deviation because stronger H-bond is formed.
The molecular weight of benzoic acid in benzene asdetermined by depression in freezing point methodcorresponds to : (1996 - 1 Mark)- a)ionization of benzoic acid.
- b)dimerization of benzoic acid.
- c)trimerization of benzoic acid.
- d)solvation of benzoic acid.
Correct answer is option 'B'. Can you explain this answer?
The molecular weight of benzoic acid in benzene asdetermined by depression in freezing point methodcorresponds to : (1996 - 1 Mark)
a)
ionization of benzoic acid.
b)
dimerization of benzoic acid.
c)
trimerization of benzoic acid.
d)
solvation of benzoic acid.
|
|
Amit Kumar answered |
Benzoic acid exists as dimer in benzene.
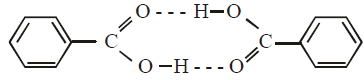
[Normal molecular mass = 122 amu
observed molecular mass = 244 amu, in case of complete association]
observed molecular mass = 244 amu, in case of complete association]
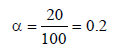
The freezing point of equimolal aqueous solutions will behighest for : (1990 - 1 Mark)- a)C6H5NH3Cl (aniline hydrochloride)
- b)Ca(NO3)2
- c)La(NO3)3
- d)C6H12O6 (glucose)
Correct answer is option 'D'. Can you explain this answer?
The freezing point of equimolal aqueous solutions will behighest for : (1990 - 1 Mark)
a)
C6H5NH3Cl (aniline hydrochloride)
b)
Ca(NO3)2
c)
La(NO3)3
d)
C6H12O6 (glucose)
|
|
Shivam Kulkarni answered |
TIPS/Formulae : The salt that ionises to least extent will have highest freezing point. [ i.e., minimum Δ Tf ]
Glucose, being non electrolyte, gives minimum no. of particles and hence minimum DTf or maximum F. pt
Glucose, being non electrolyte, gives minimum no. of particles and hence minimum DTf or maximum F. pt
The vapour pressure of water at 20° C is 17.5 mm Hg. If 18 gof glucose (C6H12O6) is added to 178.2 g of water at 20° C,the vapour pressure of the resulting solution will be [2008]- a)17.325 mm Hg
- b)15.750 mm Hg
- c)16.500 mm Hg
- d)17.500 mm Hg
Correct answer is option 'A'. Can you explain this answer?
The vapour pressure of water at 20° C is 17.5 mm Hg. If 18 gof glucose (C6H12O6) is added to 178.2 g of water at 20° C,the vapour pressure of the resulting solution will be [2008]
a)
17.325 mm Hg
b)
15.750 mm Hg
c)
16.500 mm Hg
d)
17.500 mm Hg
|
|
Rahul Bansal answered |
In solution containing nonvolatile solute,pressure is directly proportional to its mole fraction.
Psolution = vapour pressure of its pure component * mole fraction in solution
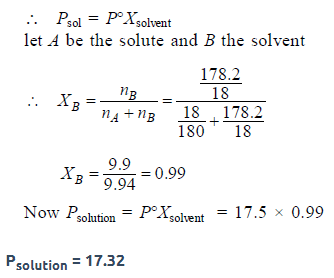
Equal masses of methane and oxygen are mixed in an emptycontainer at 25°C. The fraction of the total pressure exertedby oxygen is [2007]- a)1/2
- b)2/3
- c)
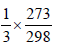
- d)1/3
Correct answer is option 'D'. Can you explain this answer?
Equal masses of methane and oxygen are mixed in an emptycontainer at 25°C. The fraction of the total pressure exertedby oxygen is [2007]
a)
1/2
b)
2/3
c)
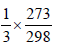
d)
1/3

|
Ayush Joshi answered |
Molar mass of methane = 16.042g mol-1
Molar mass of oxygen = 32.00 g mol-1
Therefore if say, 32g of methane and 32g of oxygen mixed,
there is 2 moles of methane and 1 moles of oxygen.
n= PV/RT
pressure is directly proportional to the number of moles
Oxygen is 1/3 of total number of moles and hence it exerts 1/3 of the total pressure
So my answer would be (D)
At 80° C, the vapour pressure of pure liquid ‘A’ is 520 mmHg and that of pure liquid ‘B’ is 1000 mm Hg. If a mixturesolution of ‘A’ and ‘B’ boils at 80° C and 1 atm pressure, theamount of ‘A’ in the mixture is (1 atm = 760 mm Hg) [2008]- a)52 mol percent
- b)34 mol percent
- c)48 mol percent
- d)50 mol percent
Correct answer is option 'D'. Can you explain this answer?
At 80° C, the vapour pressure of pure liquid ‘A’ is 520 mmHg and that of pure liquid ‘B’ is 1000 mm Hg. If a mixturesolution of ‘A’ and ‘B’ boils at 80° C and 1 atm pressure, theamount of ‘A’ in the mixture is (1 atm = 760 mm Hg) [2008]
a)
52 mol percent
b)
34 mol percent
c)
48 mol percent
d)
50 mol percent
|
|
Aryan Datta answered |
At 80, most people are considered to be in their senior years. They may have retired from work and are enjoying their leisure time. They may also be dealing with age-related health issues and may require assistance or support from family members or caregivers.
At this age, many people have accumulated a lifetime of experiences and wisdom. They may have seen significant changes in the world and have a rich knowledge of history. Some may use their time to pursue hobbies, travel, or spend time with family and friends.
It is important for individuals at this age to maintain a healthy lifestyle, including regular exercise, a balanced diet, and regular health check-ups. They may also need to adapt their living arrangements to accommodate any mobility or health issues.
Social interaction and mental stimulation are also crucial at this age. Engaging in activities that promote cognitive function, such as reading, puzzles, and socializing with peers, can help maintain mental sharpness and overall well-being.
Overall, life at 80 can vary greatly depending on the individual's health, lifestyle, and personal circumstances. While some may be active and independent, others may require more assistance and support. It is important to approach this age with a positive mindset and take steps to maintain physical and mental well-being.
At this age, many people have accumulated a lifetime of experiences and wisdom. They may have seen significant changes in the world and have a rich knowledge of history. Some may use their time to pursue hobbies, travel, or spend time with family and friends.
It is important for individuals at this age to maintain a healthy lifestyle, including regular exercise, a balanced diet, and regular health check-ups. They may also need to adapt their living arrangements to accommodate any mobility or health issues.
Social interaction and mental stimulation are also crucial at this age. Engaging in activities that promote cognitive function, such as reading, puzzles, and socializing with peers, can help maintain mental sharpness and overall well-being.
Overall, life at 80 can vary greatly depending on the individual's health, lifestyle, and personal circumstances. While some may be active and independent, others may require more assistance and support. It is important to approach this age with a positive mindset and take steps to maintain physical and mental well-being.
A 5.25% solution of a substance is isotonic with a 1.5% solutionof urea (molar mass = 60 g mol–1) in the same solvent. If thedensities of both the solutions are assumed to be equal to 1.0g cm–3, molar mass of the substance will be [2007]- a)210.0 g mol–1
- b)90.0 g mol–1
- c)115.0 g mol–1
- d)105.0 g mol–1.
Correct answer is option 'A'. Can you explain this answer?
A 5.25% solution of a substance is isotonic with a 1.5% solutionof urea (molar mass = 60 g mol–1) in the same solvent. If thedensities of both the solutions are assumed to be equal to 1.0g cm–3, molar mass of the substance will be [2007]
a)
210.0 g mol–1
b)
90.0 g mol–1
c)
115.0 g mol–1
d)
105.0 g mol–1.
|
|
Nitin Gupta answered |
Understanding Isotonic Solutions
Isotonic solutions have the same osmotic pressure. In this case, a 5.25% solution of a substance is isotonic with a 1.5% solution of urea.
Calculating Urea Molarity
- Molar mass of urea: 60 g/mol
- Density of solution: 1.0 g/cm³
- Concentration of urea: 1.5% = 1.5 g in 100 mL of solution
To find the number of moles of urea in 1.5 g:
- Moles of urea = mass (g) / molar mass (g/mol) = 1.5 g / 60 g/mol = 0.025 mol
Since 100 mL of solution has 0.025 moles of urea:
- Molarity of urea solution = 0.025 mol / 0.1 L = 0.25 M
Calculating the Molarity of the Substance
Since both solutions are isotonic:
- Molarity of the substance = Molarity of urea = 0.25 M
Finding the Molar Mass of the Substance
- Concentration of the substance: 5.25% = 5.25 g in 100 mL of solution
To find the number of moles of the substance:
- Moles of substance = mass (g) / molar mass (g/mol)
Given that the density is 1.0 g/cm³, the volume of 100 mL = 100 g (mass of the solution).
Mass of solvent in 100 mL = mass of solution - mass of solute = 100 g - 5.25 g = 94.75 g.
Using the molarity:
- Molarity = moles / volume(L) = (5.25 g / molar mass) / 0.1 L = 0.25 M
Setting the equations equal:
- (5.25 / molar mass) / 0.1 = 0.25
Solving for molar mass gives:
- molar mass = 5.25 g / (0.25 * 0.1) = 210 g/mol
Thus, the molar mass of the substance is 210 g/mol.
Conclusion
The correct answer is option 'A', 210.0 g mol–1.
Isotonic solutions have the same osmotic pressure. In this case, a 5.25% solution of a substance is isotonic with a 1.5% solution of urea.
Calculating Urea Molarity
- Molar mass of urea: 60 g/mol
- Density of solution: 1.0 g/cm³
- Concentration of urea: 1.5% = 1.5 g in 100 mL of solution
To find the number of moles of urea in 1.5 g:
- Moles of urea = mass (g) / molar mass (g/mol) = 1.5 g / 60 g/mol = 0.025 mol
Since 100 mL of solution has 0.025 moles of urea:
- Molarity of urea solution = 0.025 mol / 0.1 L = 0.25 M
Calculating the Molarity of the Substance
Since both solutions are isotonic:
- Molarity of the substance = Molarity of urea = 0.25 M
Finding the Molar Mass of the Substance
- Concentration of the substance: 5.25% = 5.25 g in 100 mL of solution
To find the number of moles of the substance:
- Moles of substance = mass (g) / molar mass (g/mol)
Given that the density is 1.0 g/cm³, the volume of 100 mL = 100 g (mass of the solution).
Mass of solvent in 100 mL = mass of solution - mass of solute = 100 g - 5.25 g = 94.75 g.
Using the molarity:
- Molarity = moles / volume(L) = (5.25 g / molar mass) / 0.1 L = 0.25 M
Setting the equations equal:
- (5.25 / molar mass) / 0.1 = 0.25
Solving for molar mass gives:
- molar mass = 5.25 g / (0.25 * 0.1) = 210 g/mol
Thus, the molar mass of the substance is 210 g/mol.
Conclusion
The correct answer is option 'A', 210.0 g mol–1.
For a dilute solution containing 2.5 g of a non-volatile nonelectrolytesolute in 100 g of water, the elevationin boiling point at 1 atm pressure is 2°C. Assumingconcentration of solute is much lower than the concentrationof solvent, the vapour pressure (mm of Hg) of the solutionis (take Kb = 0.76 K kg mol–1) (2012)- a)724
- b)740
- c)736
- d)718
Correct answer is option 'A'. Can you explain this answer?
For a dilute solution containing 2.5 g of a non-volatile nonelectrolytesolute in 100 g of water, the elevationin boiling point at 1 atm pressure is 2°C. Assumingconcentration of solute is much lower than the concentrationof solvent, the vapour pressure (mm of Hg) of the solutionis (take Kb = 0.76 K kg mol–1) (2012)
a)
724
b)
740
c)
736
d)
718
|
|
Siddharth Basak answered |
From Raoult law relation,
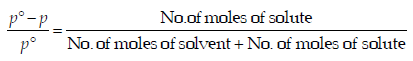
When the concentration of solute is much lower than the concentration of solvent,
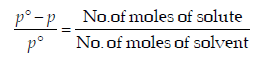
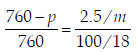 .... (i)
.... (i)From elevation in boiling point, ΔTb = Kb × m
2 = 0.76 × m
m = 2/0.76 ...(ii)
From (i) and (ii), p = 724 mm
2 = 0.76 × m
m = 2/0.76 ...(ii)
From (i) and (ii), p = 724 mm
Which of the following 0.1 M aqueous solutions will havethe lowest freezing point? (1989 - 1 Mark)- a)Potassium sulphate
- b)Sodium chloride
- c)Urea
- d)Glucose
Correct answer is option 'A'. Can you explain this answer?
Which of the following 0.1 M aqueous solutions will havethe lowest freezing point? (1989 - 1 Mark)
a)
Potassium sulphate
b)
Sodium chloride
c)
Urea
d)
Glucose
|
|
Tanuja Kapoor answered |
NOTE : The salt producing highest number of ions will have lowest freezing point.
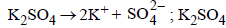 gives highest number of particles (2 + 1 = 3).
gives highest number of particles (2 + 1 = 3).Glucose, being non-electrolyte gives minimum no. of particles and hence minimum ΔTf or maximum F. pt.
The Henry’s law constant for the solubility of N2 gas inwater at 298 K is 1.0 × 105 atm. The mole fraction of N2 in airis 0.8. The number of moles of N2 from air dissolved in10 moles of water at 298 K and 5 atm pressure is (2009)- a)4.0× 10– 4
- b)4.0 × 10–5
- c)5.0 × 10– 4
- d)4.0 × 10–6
Correct answer is option 'A'. Can you explain this answer?
The Henry’s law constant for the solubility of N2 gas inwater at 298 K is 1.0 × 105 atm. The mole fraction of N2 in airis 0.8. The number of moles of N2 from air dissolved in10 moles of water at 298 K and 5 atm pressure is (2009)
a)
4.0× 10– 4
b)
4.0 × 10–5
c)
5.0 × 10– 4
d)
4.0 × 10–6

|
Ravi Varma answered |

When mercuric iodide is added to the aqueous solution ofpotassium iodide then (1987 - 1 Mark)- a)freezing point is raised.
- b)freezing point is lowered.
- c)freezing point does not change.
- d)boiling point does not change.
Correct answer is option 'A'. Can you explain this answer?
When mercuric iodide is added to the aqueous solution ofpotassium iodide then (1987 - 1 Mark)
a)
freezing point is raised.
b)
freezing point is lowered.
c)
freezing point does not change.
d)
boiling point does not change.
|
|
Kunal Nair answered |
Added HgI2 forms a complex with KI in the solution as follows
2KI + HgI2 → K2[HgI4]
As a result, number of particles decreases and so ΔTf increases.
[NOTE : : Depression in freezing point is a colligative property]
2KI + HgI2 → K2[HgI4]
As a result, number of particles decreases and so ΔTf increases.
[NOTE : : Depression in freezing point is a colligative property]
18 g of glucose (C6H12O6) is added to 178.2 g of water. Thevapour pressure of water for this aqueous solution at 100ºC is [2006]- a)76.00 Torr
- b)752.40 Torr
- c)759.00 Torr
- d)7.60 Torr
Correct answer is option 'B'. Can you explain this answer?
18 g of glucose (C6H12O6) is added to 178.2 g of water. Thevapour pressure of water for this aqueous solution at 100ºC is [2006]
a)
76.00 Torr
b)
752.40 Torr
c)
759.00 Torr
d)
7.60 Torr

|
Atharva Pillai answered |
Molecular mass of water = 2*1 + 1*16 = 18 g
For 178.2 g water, nA = 9.9
Molecular mass of glucose = 6*12 + 12*1 + 6*16 = 180 g
For 18 g glucose, nB = 0.1
XB = 0.1/(0.1+9.9) = 0.01
XA = 0.99
For lowering of vapour pressure,
P = p^0A^XA = p^0A(1 – XB)
P = 760(1 – 0.01)
= 760 - 7.6
= 752.4 torr
The correct option is B.
For which of the following parameters the structural isomersC2H5OH and CH3OCH3 would be expected to have the samevalues?(Assume ideal behaviour) [2004]- a)Boiling points
- b)Vapour pressure at the same temperature
- c)Heat of vaporization
- d)Gaseous densities at the same temperature and pressure
Correct answer is option 'D'. Can you explain this answer?
For which of the following parameters the structural isomersC2H5OH and CH3OCH3 would be expected to have the samevalues?(Assume ideal behaviour) [2004]
a)
Boiling points
b)
Vapour pressure at the same temperature
c)
Heat of vaporization
d)
Gaseous densities at the same temperature and pressure
|
|
Sanskriti Dasgupta answered |
Gaseous densities of ethanol and dimethyl ether would be same at same temperature and pressure. The heat of vaporisation, V.P. and b.pts will differ due to H-bonding in ethanol.
Ethylene glycol is used as an antifreeze in a cold climate.Mass of ethylene glycol which should be added to 4 kg ofwater to prevent it from freezing at –6°C will be : (Kf forwater = 1.86 K kg mol–1, and molar mass of ethylene glycol= 62 g mol–1) [2011]- a)804.32 g
- b)204.30 g
- c)400.00 g
- d)304.60 g
Correct answer is option 'A'. Can you explain this answer?
Ethylene glycol is used as an antifreeze in a cold climate.Mass of ethylene glycol which should be added to 4 kg ofwater to prevent it from freezing at –6°C will be : (Kf forwater = 1.86 K kg mol–1, and molar mass of ethylene glycol= 62 g mol–1) [2011]
a)
804.32 g
b)
204.30 g
c)
400.00 g
d)
304.60 g
|
|
Vaishnavi Basak answered |
Given data:
- Mass of water = 4 kg
- Freezing point of water = -6°C
- Kf for water = 1.86 K kg mol^-1
- Molar mass of ethylene glycol = 62 g mol^-1
Calculation:
1. Calculate the moles of water:
- Molar mass of water = 18 g mol^-1
- Moles of water = Mass / Molar mass = 4000 g / 18 g mol^-1 = 222.22 mol
2. Calculate the molality of the solution:
- ΔTf = Kf * m
- -6°C = 1.86 K kg mol^-1 * m
- m = -6°C / 1.86 K kg mol^-1 = -3.23 mol kg^-1
3. Calculate the moles of ethylene glycol required to prevent freezing:
- Moles of ethylene glycol = Molality * Mass of water = -3.23 mol kg^-1 * 4 kg = -12.92 mol
4. Calculate the mass of ethylene glycol required:
- Mass of ethylene glycol = Moles * Molar mass = 12.92 mol * 62 g mol^-1 = 800.32 g
Therefore, the mass of ethylene glycol that should be added to 4 kg of water to prevent it from freezing at -6°C is 804.32 g.
- Mass of water = 4 kg
- Freezing point of water = -6°C
- Kf for water = 1.86 K kg mol^-1
- Molar mass of ethylene glycol = 62 g mol^-1
Calculation:
1. Calculate the moles of water:
- Molar mass of water = 18 g mol^-1
- Moles of water = Mass / Molar mass = 4000 g / 18 g mol^-1 = 222.22 mol
2. Calculate the molality of the solution:
- ΔTf = Kf * m
- -6°C = 1.86 K kg mol^-1 * m
- m = -6°C / 1.86 K kg mol^-1 = -3.23 mol kg^-1
3. Calculate the moles of ethylene glycol required to prevent freezing:
- Moles of ethylene glycol = Molality * Mass of water = -3.23 mol kg^-1 * 4 kg = -12.92 mol
4. Calculate the mass of ethylene glycol required:
- Mass of ethylene glycol = Moles * Molar mass = 12.92 mol * 62 g mol^-1 = 800.32 g
Therefore, the mass of ethylene glycol that should be added to 4 kg of water to prevent it from freezing at -6°C is 804.32 g.
Consider separate solutions of 0.500 M C2H5OH(aq),0.100 M Mg3(PO4)2 (aq), 0.250 M KBr(aq) and 0.125 MNa3PO4(aq) at 25 C ° . Which statement is true about thesesolutions, assuming all salts to be strong electrolytes? [JEE M 2014]- a)They all have the same osmotic pressure.
- b)0.100 M Mg3(PO4)2(aq) has the highest osmoticpressure.
- c)0.125 M Na3PO4(aq) has the highest osmotic pressure.
- d)0.500 M C2H5OH(aq) has the highest osmotic pressure.
Correct answer is option 'A'. Can you explain this answer?
Consider separate solutions of 0.500 M C2H5OH(aq),0.100 M Mg3(PO4)2 (aq), 0.250 M KBr(aq) and 0.125 MNa3PO4(aq) at 25 C ° . Which statement is true about thesesolutions, assuming all salts to be strong electrolytes? [JEE M 2014]
a)
They all have the same osmotic pressure.
b)
0.100 M Mg3(PO4)2(aq) has the highest osmoticpressure.
c)
0.125 M Na3PO4(aq) has the highest osmotic pressure.
d)
0.500 M C2H5OH(aq) has the highest osmotic pressure.
|
|
Naveen Basu answered |
Understanding Osmotic Pressure
Osmotic pressure is the pressure required to stop the flow of solvent across a semipermeable membrane. It depends on the concentration of solute particles in solution.
Van't Hoff Factor (i)
For solutions of electrolytes, the osmotic pressure is calculated using the formula:
Osmotic Pressure (π) = iCRT
- i = van't Hoff factor (number of particles the solute dissociates into)
- C = molarity of the solution
- R = ideal gas constant
- T = temperature in Kelvin
Calculating for Each Solution
1. C2H5OH (0.500 M):
- Non-electrolyte, i = 1
- Effective concentration = 0.500 M
2. Mg3(PO4)2 (0.100 M):
- Strong electrolyte, i = 5 (3 Mg^2+ + 2 PO4^3-)
- Effective concentration = 0.100 M * 5 = 0.500 M
3. KBr (0.250 M):
- Strong electrolyte, i = 2 (K^+ + Br^-)
- Effective concentration = 0.250 M * 2 = 0.500 M
4. Na3PO4 (0.125 M):
- Strong electrolyte, i = 4 (3 Na^+ + PO4^3-)
- Effective concentration = 0.125 M * 4 = 0.500 M
Conclusion
- All solutions have the same effective concentration of solute particles (0.500 M).
- Therefore, they all generate the same osmotic pressure.
Thus, the correct statement is option 'A': They all have the same osmotic pressure.
Osmotic pressure is the pressure required to stop the flow of solvent across a semipermeable membrane. It depends on the concentration of solute particles in solution.
Van't Hoff Factor (i)
For solutions of electrolytes, the osmotic pressure is calculated using the formula:
Osmotic Pressure (π) = iCRT
- i = van't Hoff factor (number of particles the solute dissociates into)
- C = molarity of the solution
- R = ideal gas constant
- T = temperature in Kelvin
Calculating for Each Solution
1. C2H5OH (0.500 M):
- Non-electrolyte, i = 1
- Effective concentration = 0.500 M
2. Mg3(PO4)2 (0.100 M):
- Strong electrolyte, i = 5 (3 Mg^2+ + 2 PO4^3-)
- Effective concentration = 0.100 M * 5 = 0.500 M
3. KBr (0.250 M):
- Strong electrolyte, i = 2 (K^+ + Br^-)
- Effective concentration = 0.250 M * 2 = 0.500 M
4. Na3PO4 (0.125 M):
- Strong electrolyte, i = 4 (3 Na^+ + PO4^3-)
- Effective concentration = 0.125 M * 4 = 0.500 M
Conclusion
- All solutions have the same effective concentration of solute particles (0.500 M).
- Therefore, they all generate the same osmotic pressure.
Thus, the correct statement is option 'A': They all have the same osmotic pressure.
In the depression of freezing point experiment, it is foundthat the (1999 - 3 Marks)- a)vapour pressure of the solution is less than that ofpure solvent
- b)vapour pressure of the solution is more than that ofpure solvent
- c)only solute molecules solidify at the freezing point
- d)only solvent molecules solidify at the freezing
Correct answer is option 'A,D'. Can you explain this answer?
In the depression of freezing point experiment, it is foundthat the (1999 - 3 Marks)
a)
vapour pressure of the solution is less than that ofpure solvent
b)
vapour pressure of the solution is more than that ofpure solvent
c)
only solute molecules solidify at the freezing point
d)
only solvent molecules solidify at the freezing
|
|
Jithin Roy answered |
(a, d) The freezing point of a solvent depresses as a nonvolatile solute is added to a solvent. According to Raoult's law, when a non-volatile solute is added to a solvent the vapour pressure of the solvent decreases.
At the freezing point it will be only the solvent
molecules which will solidify.
At the freezing point it will be only the solvent
molecules which will solidify.
Chapter doubts & questions for Solutions - 35 Years Chapter wise Previous Year Solved Papers for JEE 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Solutions - 35 Years Chapter wise Previous Year Solved Papers for JEE in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup