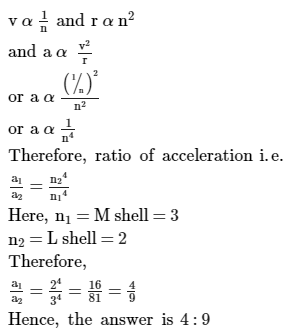All Exams >
JEE >
Physics CUET UG Mock Test Series 2026 >
All Questions
All questions of Dual Nature of Radiation and Matter for JEE Exam
Number of ejected photoelectrons increases with increase- a)never
- b)in frequency of light
- c)in wavelength of light
- d)in intensity of light
Correct answer is option 'D'. Can you explain this answer?
Number of ejected photoelectrons increases with increase
a)
never
b)
in frequency of light
c)
in wavelength of light
d)
in intensity of light
|
|
Rohan Singh answered |
A photon is the smallest possible quantum of light. In general when you turn up the intensity of light you are increasing the number of photons per second that are emitted by the light source. Therefore the intensity of the light can indeed be changed independently of the frequency (or color) of the light.
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?- a)30 V
- b)5 V
- c)10 V
- d)15 V
Correct answer is option 'C'. Can you explain this answer?
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?
a)
30 V
b)
5 V
c)
10 V
d)
15 V
|
|
Krishna Iyer answered |
Stopping potential (Vo) is given by
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in- a)wavelength of radiation
- b)intensity of radiation
- c)frequency of radiation
- d)Both the wavelength and intensity of radiation
Correct answer is option 'C'. Can you explain this answer?
In order to increase the kinetic energy of ejected photoelectrons, there should be an increase in
a)
wavelength of radiation
b)
intensity of radiation
c)
frequency of radiation
d)
Both the wavelength and intensity of radiation
|
|
Arjun Singhania answered |
The kinetic energy of emitted photoelectrons should increase with the light amplitude. The rate of electron emission, which is proportional to the measured electric current, should increase as the light frequency is increased.
Photons with energy 5eV are incident on a cathode C, on a photoelectric cell. The maximum energy of the emitted photoelectrons is 2eV. When photons of energy 6eV are incident on C, no photoelectrons will reach the anode A if the stopping potential of A relative to C is- a)3V
- b)_3V
- c)_1V
- d)4V
Correct answer is option 'B'. Can you explain this answer?
Photons with energy 5eV are incident on a cathode C, on a photoelectric cell. The maximum energy of the emitted photoelectrons is 2eV. When photons of energy 6eV are incident on C, no photoelectrons will reach the anode A if the stopping potential of A relative to C is
a)
3V
b)
_3V
c)
_1V
d)
4V
|
|
Geetika Shah answered |
When 5eV is incident the kinetic energy is 2eV it simply means the work function is W=5eV−2eV=3eV
Similarly, when 6eV is incident the kinetic energy should be 6eV−W=6eV−3eV=3eV
it simply means to stop them we need a negative potential at anode equal to 3eV/e=3V
So, the answer is −3V i.e. option B is correct.
Similarly, when 6eV is incident the kinetic energy should be 6eV−W=6eV−3eV=3eV
it simply means to stop them we need a negative potential at anode equal to 3eV/e=3V
So, the answer is −3V i.e. option B is correct.
If h is Planck's constant is SI system, the momentum of a photon of wavelength 0.01 Å is- a)10-2 h
- b)h
- c)102 h
- d)1012 h
Correct answer is option 'D'. Can you explain this answer?
If h is Planck's constant is SI system, the momentum of a photon of wavelength 0.01 Å is
a)
10-2 h
b)
h
c)
102 h
d)
1012 h
|
|
Vivek Rana answered |
Momentum of photo, p=E/c=hν/c where E is the energy of a photon and c is the velocity of light.
∴ p= hc/cλ [∵ν=λc]
p=h/λ=h/(0.01×10−10)=1012h
∴ p= hc/cλ [∵ν=λc]
p=h/λ=h/(0.01×10−10)=1012h
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes- a)Doubled
- b)Quadrupled
- c)Halved
- d)zero
Correct answer is option 'D'. Can you explain this answer?
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes
a)
Doubled
b)
Quadrupled
c)
Halved
d)
zero

|
Ayush Joshi answered |
If the frequency is halved and intensity is doubled, the frequency of incident light will become 15/2 = 0.75 times the threshold frequency. So, as ν<νo Hence, photoelectric current will be zero.
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?- a)-3.9V
- b)-8.1V
- c)-5V
- d)-1.9V
Correct answer is 'A'. Can you explain this answer?
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?
a)
-3.9V
b)
-8.1V
c)
-5V
d)
-1.9V
|
|
Priyanka Sharma answered |
From photo-electric equation, eV0= E−φ
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to- a)8 ×1014 HZ
- b)9 ×1014 HZ
- c)7 ×1014 HZ
- d)6 ×1014 HZ
Correct answer is option 'A'. Can you explain this answer?
The work function of a photoelectric material is 3.32 eV. The threshold frequency will be equal to
a)
8 ×1014 HZ
b)
9 ×1014 HZ
c)
7 ×1014 HZ
d)
6 ×1014 HZ
|
|
Anu Sharma answered |
The threshold frequency can be calculated using the formula:
threshold frequency = work function / Planck's constant
Given that the work function is 3.32 eV, we need to convert it to joules by multiplying it by the conversion factor 1.602 x 10^-19 J/eV:
work function = 3.32 eV * 1.602 x 10^-19 J/eV = 5.31264 x 10^-19 J
The value of Planck's constant is 6.626 x 10^-34 J·s.
Therefore, the threshold frequency is:
threshold frequency = 5.31264 x 10^-19 J / (6.626 x 10^-34 J·s)
threshold frequency ≈ 8.03 x 10^14 Hz
So, the threshold frequency will be approximately 8.03 x 10^14 Hz.
threshold frequency = work function / Planck's constant
Given that the work function is 3.32 eV, we need to convert it to joules by multiplying it by the conversion factor 1.602 x 10^-19 J/eV:
work function = 3.32 eV * 1.602 x 10^-19 J/eV = 5.31264 x 10^-19 J
The value of Planck's constant is 6.626 x 10^-34 J·s.
Therefore, the threshold frequency is:
threshold frequency = 5.31264 x 10^-19 J / (6.626 x 10^-34 J·s)
threshold frequency ≈ 8.03 x 10^14 Hz
So, the threshold frequency will be approximately 8.03 x 10^14 Hz.
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:- a) voltage applied to the light source
- b) intensity of light
- c) wavelength of light
- d) frequency of light
Correct answer is option 'D'. Can you explain this answer?
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:
a)
voltage applied to the light source
b)
intensity of light
c)
wavelength of light
d)
frequency of light
|
|
Rohan Singh answered |
The emission of photoelectron takes place only, when the frequency of the incident light is above a certain critical value, characteristic of that metal. The critical value of frequency is known as the threshold frequency for the metal of the emitting electrode.
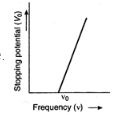
Suppose that when light of certain frequency is incident over a metal surface, the photo- electrons are emitted. To take photoelectric current zero, a particular value of stopping potential will be needed. If we go on reducing the frequency of incident light, the value of stopping potential will also go on decreasing. At certain value of frequency v0, the photoelectric current will become zero, even when no retarding potential is applied. This frequency vq corresponds to the threshold for the metal surface. The emission of photoelectrons does not take place, till frequency of incident light is below this value.

Can you explain the answer of this question below:Light from a bulb is falling on a wooden table but no photo electrons are emitted as
- A:
Work function of wood is less
- B:
Work function of wood is more
- C:
It depends on the frequency
- D:
It is independent of work function
The answer is b.
Light from a bulb is falling on a wooden table but no photo electrons are emitted as
Work function of wood is less
Work function of wood is more
It depends on the frequency
It is independent of work function
|
|
Rishika Patel answered |
Explanation:
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
In an atom, two electrons move around the nucleus in circular orbits of radii R and 4R. The ratio of the time taken by them to complete one revolution is : (neglect electric interaction)- a)1 : 4
- b)4 : 1
- c)1 : 8
- d) 8 : 1
Correct answer is option 'C'. Can you explain this answer?
In an atom, two electrons move around the nucleus in circular orbits of radii R and 4R. The ratio of the time taken by them to complete one revolution is : (neglect electric interaction)
a)
1 : 4
b)
4 : 1
c)
1 : 8
d)
8 : 1
|
|
Riya Banerjee answered |
Time period, T∝(R)3/2
∴ T1/T2 = (R1/R2 )3/2= (1/4)3/2=1/8
Thus, the ratio of time period is 1:8
∴ T1/T2 = (R1/R2 )3/2= (1/4)3/2=1/8
Thus, the ratio of time period is 1:8
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is- a)total momentum
- b)number of photons
- c)total energy
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
In a photon-particle collision (such as photon-electron collision) the quantity which is not conserved is
a)
total momentum
b)
number of photons
c)
total energy
d)
None of the above
|
|
Rohan Singh answered |
Energy and momentum are conserved, resulting in a reduction of both for the scattered photon. ... This phenomenon could be handled as a collision between two particles—a photon and an electron at rest in the material. Energy and momentum are conserved in the collision.
Difference between nth and (n + 1)th Bohr's radius of `H' atom is equal to it's (n _ 1)th Bohr's radius. The value of n is- a)1
- b)2
- c)3
- d)4
Correct answer is option 'D'. Can you explain this answer?
Difference between nth and (n + 1)th Bohr's radius of `H' atom is equal to it's (n _ 1)th Bohr's radius. The value of n is
a)
1
b)
2
c)
3
d)
4
|
|
Rajesh Gupta answered |
We know that rn∝n2
Given: rn+1−rn=rn−1
∴(n+1)2−n2=(n−1)2
n=4
Given: rn+1−rn=rn−1
∴(n+1)2−n2=(n−1)2
n=4
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is- a)Greater with visible light
- b)Equal in both cases
- c)Greater with ultraviolet light
- d)Infinite in both cases
Correct answer is 'C'. Can you explain this answer?
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is
a)
Greater with visible light
b)
Equal in both cases
c)
Greater with ultraviolet light
d)
Infinite in both cases
|
|
Riya Banerjee answered |
λ for U.V is less than λ for visible light
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
In an experiment of photoelectric emission for incident light of 4000 A0, the stopping potential is 2V. If the wavelength of incident light is made 3000 A0, then stopping potential will be- a)zero
- b)more than 2 volt
- c)2 Volt
- d)less than 2 volt
Correct answer is option 'B'. Can you explain this answer?
In an experiment of photoelectric emission for incident light of 4000 A0, the stopping potential is 2V. If the wavelength of incident light is made 3000 A0, then stopping potential will be
a)
zero
b)
more than 2 volt
c)
2 Volt
d)
less than 2 volt
|
|
Geetika Shah answered |
The maximum kinetic energy for the photoelectrons is
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
If Vo is the stopping potential then
eV0=h(c/λ)−ϕ .....................(since, ν=c/λ)
As per the problem, for incident light of 4000Ao, the stopping potential is 2V. When the wavelength of incident light is reduced to 3000Ao, then the stopping potential will increase to value more than 2V(as per the above equation).
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
If Vo is the stopping potential then
eV0=h(c/λ)−ϕ .....................(since, ν=c/λ)
As per the problem, for incident light of 4000Ao, the stopping potential is 2V. When the wavelength of incident light is reduced to 3000Ao, then the stopping potential will increase to value more than 2V(as per the above equation).
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
- a)2.2 x 10-19 J
- b)1.75 x 10-19 J
- c)0.75 x 10-19 J
- d)1.1 x 10-19 J
Correct answer is option 'B'. Can you explain this answer?
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
a)
2.2 x 10-19 J
b)
1.75 x 10-19 J
c)
0.75 x 10-19 J
d)
1.1 x 10-19 J
|
|
Om Desai answered |
Given,
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
A parallel beam of uniform, monochromatic light of wavelength 2640 A has an intensity of 200 W/m2. The number of photons in 1 mm3 of this radiation are ...............
Correct answer is '885'. Can you explain this answer?
A parallel beam of uniform, monochromatic light of wavelength 2640 A has an intensity of 200 W/m2. The number of photons in 1 mm3 of this radiation are ...............
|
|
Rajesh Gupta answered |
I=nhv/ΔtA=[N.h(C/ λ)]/(1mm/c)x(1mm2)
N=200x10-9x2640x10-10/(3x108)2x6.63x10-34
=885 Photons/mm3
N=200x10-9x2640x10-10/(3x108)2x6.63x10-34
=885 Photons/mm3
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is- a)1.64 eV
- b)1.56 eV
- c)1.52 eV
- d)1.41 eV
Correct answer is option 'A'. Can you explain this answer?
Wavelength of light incident on a photo cell is 3000 Â, if stopping potential is 2.5 volts, then work function of the cathode of photo cell is
a)
1.64 eV
b)
1.56 eV
c)
1.52 eV
d)
1.41 eV
|
|
Neha Sharma answered |
The Stopping potential =2.5V.
or, Kinetic energy=2.5eV.
We know that,
Incident energy =work function + Kinetic energy.
To get incident energy in e.V,
We also know that,
The Incident energy =12400/λ Å
Incident energy=work function + kinetic energy.
12400/3000 = work function + 2.5e.v.
4.13-2.5 = work function
work function=1.64 e.V
or, Kinetic energy=2.5eV.
We know that,
Incident energy =work function + Kinetic energy.
To get incident energy in e.V,
We also know that,
The Incident energy =12400/λ Å
Incident energy=work function + kinetic energy.
12400/3000 = work function + 2.5e.v.
4.13-2.5 = work function
work function=1.64 e.V
In which case is electron emission from a metal not known?- a)Illuminating a metal surface with suitable light
- b)Applying a very strong magnetic field to a metal
- c)Applying a very strong electric field to a metal
- d)Heating the metal sufficiently
Correct answer is option 'B'. Can you explain this answer?
In which case is electron emission from a metal not known?
a)
Illuminating a metal surface with suitable light
b)
Applying a very strong magnetic field to a metal
c)
Applying a very strong electric field to a metal
d)
Heating the metal sufficiently

|
Divey Sethi answered |
The correct answer would be option B. Applying a very strong magnetic field.
Applying a very strong magnetic field to a metal we don’t know the electron emission.
Applying a very strong magnetic field to a metal we don’t know the electron emission.
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is- a)1.6 ×1013Hz
- b)1.5 ×1013Hz
- c)1.7 ×1013Hz
- d)1.8 ×1013Hz
Correct answer is option 'B'. Can you explain this answer?
Given h = 6.6 ×10−34 joule sec, the momentum of each photon in a given radiation is 3.3 ×10−29 kg metre/sec. The frequency of radiation is
a)
1.6 ×1013Hz
b)
1.5 ×1013Hz
c)
1.7 ×1013Hz
d)
1.8 ×1013Hz
|
|
Lavanya Menon answered |
Frequency=C/ λ
λ=h/P
frequency=C P/ h
f=(3×108×3.3×10-29)/6.6×10-34
f=3× 1013/2
f=1.5×1013 Hz
λ=h/P
frequency=C P/ h
f=(3×108×3.3×10-29)/6.6×10-34
f=3× 1013/2
f=1.5×1013 Hz
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?- a)
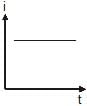
- b)
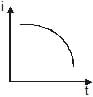
- c))

- d)
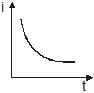
Correct answer is option 'D'. Can you explain this answer?
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?
a)
b)
c)
) 
d)
|
|
Geetika Shah answered |
Saturation current is the maximum current possible and it will be directly proportional to the number of number of electrons falling on collector plate per second which depend on number of photons
incident on the cathode as one photon contribute in one electron and the number of photons is actually
proportional to intensity which varies
As intensity I∝1/r2, where r is the distance
So the correct graph will be decreasing with power2 of distance and it will be rapidly decreasing with a higher value of r.
incident on the cathode as one photon contribute in one electron and the number of photons is actually
proportional to intensity which varies
As intensity I∝1/r2, where r is the distance
So the correct graph will be decreasing with power2 of distance and it will be rapidly decreasing with a higher value of r.
Cut off potentials for a metal in photoelectric effect for light of wavelength l1, l2 and l3 is found to be V1, V2 and V3 volts if V1, V2 and V3 are in Arithmetic Progression and l1, l2 and l3 will be- a)Arithmetic Progression
- b)Geometric Progression
- c)Harmonic Progression
- d)None
Correct answer is option 'C'. Can you explain this answer?
Cut off potentials for a metal in photoelectric effect for light of wavelength l1, l2 and l3 is found to be V1, V2 and V3 volts if V1, V2 and V3 are in Arithmetic Progression and l1, l2 and l3 will be
a)
Arithmetic Progression
b)
Geometric Progression
c)
Harmonic Progression
d)
None

|
Divey Sethi answered |
We know that,
eV=(hc/λ)-w
V=(hc/eλ)-(w/e)
Arithmetic progression =>V2=(V1+V2)/2
Now,
(hc/eλ2)-w/e=1/2[(hc/eλ1)-(w/e) +(hc/eλ3) -(w/e)]
=>1/ λ2=1/2[(1/ λ1)+(1/λ3)]
=>2/ λ2=1/ λ1 + 1/λ3
Hence the correct answer is harmonic Progression.
eV=(hc/λ)-w
V=(hc/eλ)-(w/e)
Arithmetic progression =>V2=(V1+V2)/2
Now,
(hc/eλ2)-w/e=1/2[(hc/eλ1)-(w/e) +(hc/eλ3) -(w/e)]
=>1/ λ2=1/2[(1/ λ1)+(1/λ3)]
=>2/ λ2=1/ λ1 + 1/λ3
Hence the correct answer is harmonic Progression.
A image of the sun of formed by a lens of focal-length of 30 cm on the metal surface of a photo-electric cell and a photo-electrci current is produced. The lens forming the image is then replaced by another of the same diameter but of focal length 15 cm. The photo-electric current in this case is- a)I/2
- b)I
- c)2I
- d)4I
Correct answer is option 'B'. Can you explain this answer?
A image of the sun of formed by a lens of focal-length of 30 cm on the metal surface of a photo-electric cell and a photo-electrci current is produced. The lens forming the image is then replaced by another of the same diameter but of focal length 15 cm. The photo-electric current in this case is
a)
I/2
b)
I
c)
2I
d)
4I

|
Knowledge Hub answered |
Lenses of the same diameter collect equal amounts of light.
Intensity is the measure of amount of light collected, hence the intensity remains the same.
Intensity is measured by the photoelectric current. So the photoelectric current would also remain the same.
Intensity is the measure of amount of light collected, hence the intensity remains the same.
Intensity is measured by the photoelectric current. So the photoelectric current would also remain the same.
The stopping potential for the photo electrons emitted from a metal surface of work function 1.7eV is 10.4 V. Identify the energy levels corresponding to the transitions in hydrogen atom which will result in emission of wavelength equal to that of incident radiation for the above photoelectric effect- a)n = 3 to 1
- b)n = 3 to 2
- c)n = 2 to 1
- d)n = 4 to 1
Correct answer is option 'A'. Can you explain this answer?
The stopping potential for the photo electrons emitted from a metal surface of work function 1.7eV is 10.4 V. Identify the energy levels corresponding to the transitions in hydrogen atom which will result in emission of wavelength equal to that of incident radiation for the above photoelectric effect
a)
n = 3 to 1
b)
n = 3 to 2
c)
n = 2 to 1
d)
n = 4 to 1
|
|
Gaurav Kumar answered |
As we know that the stopping potential of the photoelectron is equal to the maximum kinetic energy of the photoelectron,
KEmax=10.4V
Now, in photoelectric effect,
Energy of incident radiation (Ein) = work function + K.Emax
⇒ Ein=1.7+10.4
⇒ Ein=12.1eV
Now, for 0 hydrogen atom,
Energy of first energy level, E1=−13.6eV
Energy of second energy level, E2=−3.4eV
Energy of third energy level, E3=−1.5eV
Hence, a transition from third to first energy level will result in emission of radiation of energy = E3−E1=12.1eV which is same as the energy of incident radiation of above photoelectric effect.
Thus, correct answer is n=3 to 1
KEmax=10.4V
Now, in photoelectric effect,
Energy of incident radiation (Ein) = work function + K.Emax
⇒ Ein=1.7+10.4
⇒ Ein=12.1eV
Now, for 0 hydrogen atom,
Energy of first energy level, E1=−13.6eV
Energy of second energy level, E2=−3.4eV
Energy of third energy level, E3=−1.5eV
Hence, a transition from third to first energy level will result in emission of radiation of energy = E3−E1=12.1eV which is same as the energy of incident radiation of above photoelectric effect.
Thus, correct answer is n=3 to 1
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is- a)2 V
- b)6 V
- c)4 V
- d)10 V
Correct answer is option 'C'. Can you explain this answer?
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential is
a)
2 V
b)
6 V
c)
4 V
d)
10 V
|
|
Rajeev Saxena answered |
Stopping potential is nothing but the maximum kinetic energy of electros which get emitted during photoelectric effect.
So the stopping potential = maximum kinetic energy = 4eV
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4eV. The stopping potential is Volts is- a)2
- b)4
- c)6
- d)10
Correct answer is option 'B'. Can you explain this answer?
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4eV. The stopping potential is Volts is
a)
2
b)
4
c)
6
d)
10
|
|
Naina Datta answered |
Explanation:
When a photon of energy hν falls on the surface of a metal, an electron is emitted if the energy of the photon is greater than or equal to the work function of the metal.
The maximum kinetic energy of the emitted photoelectrons is given by:
K.E. max = hν - φ
where h is Planck’s constant, ν is the frequency of the incident radiation, and φ is the work function of the metal.
Given that the energy of the incident photons is 6 eV and the maximum kinetic energy of the emitted photoelectrons is 4 eV, we have:
K.E. max = 4 eV
hν = 6 eV
Substituting these values in the above equation, we get:
4 eV = 6 eV - φ
φ = 2 eV
Therefore, the work function of the metal is 2 eV.
When a potential difference (stopping potential) V is applied between the metal surface and the collector electrode, the photoelectrons are brought to rest and the maximum kinetic energy of the emitted photoelectrons is equal to the potential energy gained by the electrons in moving from the metal surface to the collector electrode.
Therefore, we have:
K.E. max = eV
where e is the electronic charge.
Substituting the value of K.E. max and e in the above equation, we get:
4 eV = eV
V = 4 V
Hence, the stopping potential is 4 volts. Therefore, option B is the correct answer.
When a photon of energy hν falls on the surface of a metal, an electron is emitted if the energy of the photon is greater than or equal to the work function of the metal.
The maximum kinetic energy of the emitted photoelectrons is given by:
K.E. max = hν - φ
where h is Planck’s constant, ν is the frequency of the incident radiation, and φ is the work function of the metal.
Given that the energy of the incident photons is 6 eV and the maximum kinetic energy of the emitted photoelectrons is 4 eV, we have:
K.E. max = 4 eV
hν = 6 eV
Substituting these values in the above equation, we get:
4 eV = 6 eV - φ
φ = 2 eV
Therefore, the work function of the metal is 2 eV.
When a potential difference (stopping potential) V is applied between the metal surface and the collector electrode, the photoelectrons are brought to rest and the maximum kinetic energy of the emitted photoelectrons is equal to the potential energy gained by the electrons in moving from the metal surface to the collector electrode.
Therefore, we have:
K.E. max = eV
where e is the electronic charge.
Substituting the value of K.E. max and e in the above equation, we get:
4 eV = eV
V = 4 V
Hence, the stopping potential is 4 volts. Therefore, option B is the correct answer.
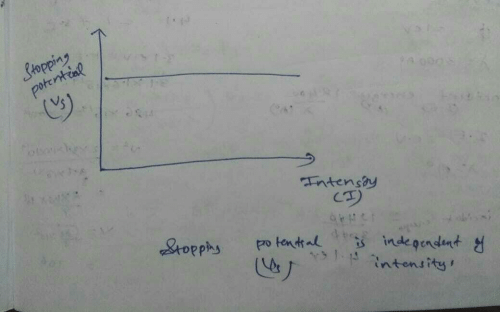
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation- a)is independent of the radiation intensity
- b)is inversely proportional to radiation intensity
- c)is proportional to radiation intensity
- d)none
Correct answer is option 'A'. Can you explain this answer?
In various experiments on photo electricity the stopping potential for a given frequency of the incident radiation
a)
is independent of the radiation intensity
b)
is inversely proportional to radiation intensity
c)
is proportional to radiation intensity
d)
none
|
|
Shreya Singh answered |
If the frequency of light in a photoelectric experiment is doubled, the stopping potential will- a)be doubled
- b)halved
- c)become more than doubled
- d)become less than double
Correct answer is option 'C'. Can you explain this answer?
If the frequency of light in a photoelectric experiment is doubled, the stopping potential will
a)
be doubled
b)
halved
c)
become more than doubled
d)
become less than double
|
|
Om Desai answered |
The maximum kinetic energy for the photoelectrons is
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
eV0=hν−ϕ ...................(1)
where, V0 is the stopping potential and e is the electronic charge.
When, the frequency of light in a photoelectric experiment is doubled,
eV0′=2hν−ϕ
eV0′=2[hν−(ϕ/2)].........................(2)
From the above two equations we can say that the K.E. in (2) is more than double of K.E in (1). Hence, when the frequency of light in a photoelectric experiment is doubled, the stopping potential becomes more than double.
So, the answer is option (C).
Emax=hν−ϕ
where, ν is the frequency of incident light and ϕ is photoelectric work function of metal.
eV0=hν−ϕ ...................(1)
where, V0 is the stopping potential and e is the electronic charge.
When, the frequency of light in a photoelectric experiment is doubled,
eV0′=2hν−ϕ
eV0′=2[hν−(ϕ/2)].........................(2)
From the above two equations we can say that the K.E. in (2) is more than double of K.E in (1). Hence, when the frequency of light in a photoelectric experiment is doubled, the stopping potential becomes more than double.
So, the answer is option (C).
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is- a)1:5
- b)1:4
- c)1:3
- d)1:2
Correct answer is option 'D'. Can you explain this answer?
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is
a)
1:5
b)
1:4
c)
1:3
d)
1:2
|
|
Shreya Gupta answered |
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is- a)7 ×10-17 J
- b)8 ×10−17 J
- c)6 ×10−17 J
- d)9 ×10−17 J
Correct answer is option 'B'. Can you explain this answer?
If the voltage across the electrodes of a cathode ray tube is 500 volts then energy gained by the electrons is
a)
7 ×10-17 J
b)
8 ×10−17 J
c)
6 ×10−17 J
d)
9 ×10−17 J
|
|
Om Desai answered |
Voltage across the electrodes of a cathode ray gun, V=500V
Charge of the electron=1.6x10-19
Energy=eV
E= 1.6x10-19 x 500
E=800 x 10-19 J
E=8 x 10-17 J
Charge of the electron=1.6x10-19
Energy=eV
E= 1.6x10-19 x 500
E=800 x 10-19 J
E=8 x 10-17 J
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :- a) n1 = 4, n2 = 2
- b)n1 = 8, n2 = 2
- c)n1 = 8, n2 = 1
- d)n1 = 6, n2 = 3
Correct answer is option 'A,D'. Can you explain this answer?
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :
a)
n1 = 4, n2 = 2
b)
n1 = 8, n2 = 2
c)
n1 = 8, n2 = 1
d)
n1 = 6, n2 = 3
|
|
Om Desai answered |
For an electron revolving in nth orbit around the nucleus of hydrogen atom, Fcentrifugal=Fcoulomb
Thus, mvn2/ rn=e2/4πϵorn2
Also, mvnrn=nh/2π
On solving these two we get: vn= e21/2ϵohn and rn=( ϵoh2/πme2 ) n2
Time period, T=2πrn/vn⟹T∝n3
Thus, 8T/T=(n1/n2)3⟹n1=2n2
Thus, n1=4,n2=2 and n1=6,n2=3
Thus, mvn2/ rn=e2/4πϵorn2
Also, mvnrn=nh/2π
On solving these two we get: vn= e21/2ϵohn and rn=( ϵoh2/πme2 ) n2
Time period, T=2πrn/vn⟹T∝n3
Thus, 8T/T=(n1/n2)3⟹n1=2n2
Thus, n1=4,n2=2 and n1=6,n2=3
Photons can be- a)scattered
- b)deflected by magnetic fields
- c)deflected by electric fields
- d)none
Correct answer is option 'A'. Can you explain this answer?
Photons can be
a)
scattered
b)
deflected by magnetic fields
c)
deflected by electric fields
d)
none
|
|
Rajeev Saxena answered |
As this electron changes orbit, its energy is reduced, and the excess energy is given off in the form of a photon, called a “characteristic photon.” In pair production, photon energies greater than 1.02 MeV interact with the strong electric field of the nucleus and lose all incident energy.
In the experiment on photoelectric effect using light having frequency greater than the threshold frequency, the photocurrent will certainly increase when- a)Anode voltage is incrased
- b)Area of cathode surface is increased
- c)Intensity of incident light is increased
- d)Distance between anode and cathode is increased.
Correct answer is option 'B,C'. Can you explain this answer?
In the experiment on photoelectric effect using light having frequency greater than the threshold frequency, the photocurrent will certainly increase when
a)
Anode voltage is incrased
b)
Area of cathode surface is increased
c)
Intensity of incident light is increased
d)
Distance between anode and cathode is increased.
|
|
Riya Banerjee answered |
If light of certain frequency, greater than the threshold frequency, is incident of the metal surface the photoelectric current obtained is directly proportional to the number of photo-electrons emitted. On increasing the frequency of incident light (or) by increasing the anode potential the speed of photo-electrons emitted changes but they have no effect on the number of electrons emitted. But by increasing the intensity of the incident light more photo-electrons will be emitted and thereby increasing the photoelectric current.
Also, if we increase the area on which the light is incident we can get more electrons emitted which increases the photoelectric current.
Also, if we increase the area on which the light is incident we can get more electrons emitted which increases the photoelectric current.
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then findQ. the saturation current
Correct answer is '2.0'. Can you explain this answer?
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then find
Q.
the saturation current
|
|
Neha Sharma answered |
Intensity of photons ↑, no. of electron ↑
The cut off voltage does not depend on distance between cell and source.
But intensity is inversely proportional to square of it's distance.
∴ at d=0.6m,
Cut off voltage =0.6V
but
Is=18×(0.2/0.6)2
=2mA
So, the answers are option 2.0 mA
The cut off voltage does not depend on distance between cell and source.
But intensity is inversely proportional to square of it's distance.
∴ at d=0.6m,
Cut off voltage =0.6V
but
Is=18×(0.2/0.6)2
=2mA
So, the answers are option 2.0 mA
In Photoelectric effect- a)electrical energy is converted magnetic field energy
- b)electrical energy is converted into light energy
- c)light is converted into electrical energy
- d)electrical energy is converted into heat
Correct answer is option 'C'. Can you explain this answer?
In Photoelectric effect
a)
electrical energy is converted magnetic field energy
b)
electrical energy is converted into light energy
c)
light is converted into electrical energy
d)
electrical energy is converted into heat
|
|
Nikita Singh answered |
Photoelectric cell or photocell, device whose electrical characteristics (e.g., current, voltage, or resistance) vary when light is incident upon it. The most common type consists of two electrodes separated by a light-sensitive semiconductor material.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner can be called photoelectrons.
A photon is- a)a positive charged particle
- b)a quantum of light energy
- c)an instrument for measuring light intensity
- d)a quantum of matter
Correct answer is option 'B'. Can you explain this answer?
A photon is
a)
a positive charged particle
b)
a quantum of light energy
c)
an instrument for measuring light intensity
d)
a quantum of matter

|
Knowledge Hub answered |
A particle representing a quantum of light or other electromagnetic radiation. A photon carries energy proportional to the radiation frequency but has zero rest mass.
By increasing the intensity of incident light keeping frequency (v > v0) fixed on the surface of metal- a)kinetic energy of the photoelectrons increases
- b)number of emitted electrons increases
- c)kinetic energy and number of electrons increases
- d)no effect
Correct answer is option 'B'. Can you explain this answer?
By increasing the intensity of incident light keeping frequency (v > v0) fixed on the surface of metal
a)
kinetic energy of the photoelectrons increases
b)
number of emitted electrons increases
c)
kinetic energy and number of electrons increases
d)
no effect
|
|
Geetika Shah answered |
The number of photoelectrons emitted per second from a photosensitive plate is directly proportional to the intensity of the incident radiation. ... For the same frequency of light and increased intensity, the saturation current is found to increase, but the cut-off potential is found to remain constant.
The number of electrons also changes because of the probability that each photon results in an emitted electron are a function of photon energy. If the intensity of the incident radiation of a given frequency is increased, there is no effect on the kinetic energy of each photo electron.
The number of electrons also changes because of the probability that each photon results in an emitted electron are a function of photon energy. If the intensity of the incident radiation of a given frequency is increased, there is no effect on the kinetic energy of each photo electron.
The radius of Bohr's first orbit is a0. The electron in nth orbit has a radius- a)na0
- b)a0/n
- c)n2a0
- d)a0/n2
Correct answer is option 'C'. Can you explain this answer?
The radius of Bohr's first orbit is a0. The electron in nth orbit has a radius
a)
na0
b)
a0/n
c)
n2a0
d)
a0/n2
|
|
Gaurav Kumar answered |
Radius of nth orbital
rn= ϵ0n2h2/ πmZe2
Wherein
=rn∝ n2 /Z
= ϵ0h2/πme2 =0.529
r=(n2/Z)a0
For Z=1 r=n2a0
rn= ϵ0n2h2/ πmZe2
Wherein
=rn∝ n2 /Z
= ϵ0h2/πme2 =0.529
r=(n2/Z)a0
For Z=1 r=n2a0
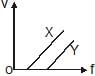
In a photoelectric experiment, electrons are ejected from metals X and Y by light of intensity I and frequency f. The potential difference V required to stop the electrons is measured for various frequencies. If Y has a greater work function than X ; which one of the following graphs best illustrates the expected results ?- a)
- b)
- c)
- d)

Correct answer is option 'A'. Can you explain this answer?
In a photoelectric experiment, electrons are ejected from metals X and Y by light of intensity I and frequency f. The potential difference V required to stop the electrons is measured for various frequencies. If Y has a greater work function than X ; which one of the following graphs best illustrates the expected results ?
a)
b)
c)
d)
|
|
Nikita Singh answered |
The answer is option A.
First, the gradient of the graph cannot change (always = h/e ), so answers are A or D.
If Y has a greater work function than X, then the graph for Y should have a more negative y-intercept. therefore figure in option A depicts the concept.
First, the gradient of the graph cannot change (always = h/e ), so answers are A or D.
If Y has a greater work function than X, then the graph for Y should have a more negative y-intercept. therefore figure in option A depicts the concept.
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 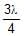 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :- a)3K/4
- b)4K/3
- c)Less than 4K/3
- d)Greater than 4K/3
Correct answer is option 'D'. Can you explain this answer?
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 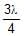 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :
a)
3K/4
b)
4K/3
c)
Less than 4K/3
d)
Greater than 4K/3
|
|
Lavanya Menon answered |
From E=W0+(1/2)mvmax2⇒vmax= √[(2E/m)−(2W0/m)]
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
where E= hc/λ
If wavelength of incident light charges from λ to 3λ/4 (Decreases)
Let energy of incident light charges from E and speed of fastest electron changes from v to v′ then
v′=√[(2E′/m)−(2W0/m)]
As E∝1/λ⇒E′(4/3)E
Hence v′=√[(2(4/3)E/m)−(2W0/m)]
⇒v′=(4/3)1/2 [(2E/m)− (2W0/m(4/3)1/2)]
So, v′>(4/3)1/2v
in photoelectric effect, the photoelectric current- a)both on intensity and frequency of incident beam
- b)increases when frequency of incident photons increases
- c)decreases when frequency of incident photons increases
- d)does not depend on photon frequency but only on intensity of incident
Correct answer is option 'D'. Can you explain this answer?
in photoelectric effect, the photoelectric current
a)
both on intensity and frequency of incident beam
b)
increases when frequency of incident photons increases
c)
decreases when frequency of incident photons increases
d)
does not depend on photon frequency but only on intensity of incident
|
|
Arun Khanna answered |
In photoelectric effect,when light incident on metal surface the no. Of electron emitted is depends on intensity of light and speed of Electron is depends on frequency of incidents light. Photoelectric current is due to the ejection of photo electrons.
An electron is in an excited state in hydrogen-like atom. It has a total energy of –3.4 eV. If the kinetic energy of the electron is E and its de-Broglie wavelength is l, then- a)E = 6.8 eV, l = 6.6 × 10–10 m
- b)E = 3.4 eV, l = 6.6 × 10–10 m
- c)E = 3.4 eV, l = 6.6 × 10–11 m
- d)E = 6.8 eV, l = 6.6 × 10–11 m
Correct answer is option 'B'. Can you explain this answer?
An electron is in an excited state in hydrogen-like atom. It has a total energy of –3.4 eV. If the kinetic energy of the electron is E and its de-Broglie wavelength is l, then
a)
E = 6.8 eV, l = 6.6 × 10–10 m
b)
E = 3.4 eV, l = 6.6 × 10–10 m
c)
E = 3.4 eV, l = 6.6 × 10–11 m
d)
E = 6.8 eV, l = 6.6 × 10–11 m
|
|
Hansa Sharma answered |
The potential energy of an electron is twice the kinetic energy(with negative sign) of an electron.
PE=2E
The total energy is: TE=PE+KE
−3.4=−2×3.4+KE
KE=3.4eV
Let p be the momentum of an electron and m be the mass of an electron.
E=p2/2m
p=√2mE
Now, the De-Broglie wavelength associated with an electron is:
λ=h/p
λ=h/√2mE
λ=6.6×1034/√2×9.1×10−31×(−3.4)×1.6×10−19
λ=6.6×1034/9.95×10−25
0λ=0.66×10−9
λ=6.6×10−10m
PE=2E
The total energy is: TE=PE+KE
−3.4=−2×3.4+KE
KE=3.4eV
Let p be the momentum of an electron and m be the mass of an electron.
E=p2/2m
p=√2mE
Now, the De-Broglie wavelength associated with an electron is:
λ=h/p
λ=h/√2mE
λ=6.6×1034/√2×9.1×10−31×(−3.4)×1.6×10−19
λ=6.6×1034/9.95×10−25
0λ=0.66×10−9
λ=6.6×10−10m
663 mW of light from a 540 nm source is incident on the surface of a metal. If only 1 of each 5×109 incident photons in absorbed and causes an electron to be ejected from the surface, the total photocurrent in the circuit is ____________________.- a)5.76 x 1011
- b)5.76 x 109
- c)5.76 x 1010
- d)5.76 x 1012
Correct answer is option 'A'. Can you explain this answer?
663 mW of light from a 540 nm source is incident on the surface of a metal. If only 1 of each 5×109 incident photons in absorbed and causes an electron to be ejected from the surface, the total photocurrent in the circuit is ____________________.
a)
5.76 x 1011
b)
5.76 x 10
9
c)
5.76 x 1010
d)
5.76 x 1012
|
|
Suresh Iyer answered |
N / Δt = no. of photon incident per second.
∴ 663x10-3=(N/Δt)x(hc/λ)
∴N/ Δt=663x10-3/(hc/λ)= 663x10-3/(1242nmeV/540)
(n/Δt)/(N/Δt)=1/(5x109)
n/Δt=[1/(5x109)]x (N/Δt)
=[1/(5x109)]x ((663x10-3x540)/1242nmeV)
I=ne/Δt=5.76x1011A
∴ 663x10-3=(N/Δt)x(hc/λ)
∴N/ Δt=663x10-3/(hc/λ)= 663x10-3/(1242nmeV/540)
(n/Δt)/(N/Δt)=1/(5x109)
n/Δt=[1/(5x109)]x (N/Δt)
=[1/(5x109)]x ((663x10-3x540)/1242nmeV)
I=ne/Δt=5.76x1011A
Chapter doubts & questions for Dual Nature of Radiation and Matter - Physics CUET UG Mock Test Series 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Dual Nature of Radiation and Matter - Physics CUET UG Mock Test Series 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup