All Exams >
NEET >
Chemistry CUET UG Mock Test Series 2026 >
All Questions
All questions of Electrochemistry for NEET Exam
Molar conductivity for a compound AB is 145.0 Scm2mol-1 and for CB is 110.1 Scm2mol-1. Limiting molar conductivity for A+ is 73.5 Scm2mol-1. What is limiting molar conductivity for C+?- a)326.6 S cm2 mol-1
- b)38.6 S cm2 mol-1
- c)181.6 S cm2 mol-1
- d)90.8 S cm2 mol-1
Correct answer is option 'B'. Can you explain this answer?
Molar conductivity for a compound AB is 145.0 Scm2mol-1 and for CB is 110.1 Scm2mol-1. Limiting molar conductivity for A+ is 73.5 Scm2mol-1. What is limiting molar conductivity for C+?
a)
326.6 S cm2 mol-1
b)
38.6 S cm2 mol-1
c)
181.6 S cm2 mol-1
d)
90.8 S cm2 mol-1

|
Priyal answered |

Chemical used in salt bridge isa. KOHb. KCIc. KNO2d. KBrCorrect answer is option 'B'. Can you explain this answer?
|
|
Anand Saha answered |
KCl is used as salt bridge because it provides positive K+ ions and negative Cl- ions as the salt bridge needs to maintain the neutrality in the system by providing enough negative ions equal to the positive ions during oxidation.
The reduction potential of an element A is -2.71V.What can be concluded from this?- a)A will be a good oxidising agent
- b)A will accept electrons easily
- c)A will undergo reduction easily
- d)A will undergo oxidation easily
Correct answer is 'D'. Can you explain this answer?
The reduction potential of an element A is -2.71V.What can be concluded from this?
a)
A will be a good oxidising agent
b)
A will accept electrons easily
c)
A will undergo reduction easily
d)
A will undergo oxidation easily
|
|
Avantika Dasgupta answered |
Reduction Potential of Element A
The reduction potential of an element A is -2.71V. This means that the element A has a strong tendency to undergo oxidation and lose electrons. The more negative the reduction potential value, the easier it is for the element to undergo oxidation and lose electrons.
Explanation of Options
a) A will be a good oxidising agent - This statement is incorrect. A good oxidizing agent is one that accepts electrons from other species and undergoes reduction. But, since the reduction potential of element A is negative, it indicates that the element A is likely to undergo oxidation and lose electrons, so it is not a good oxidizing agent.
b) A will accept electrons easily - This statement is incorrect. The reduction potential value is negative, which means that the element A has a strong tendency to lose electrons and undergo oxidation, rather than accepting electrons and undergoing reduction.
c) A will undergo reduction easily - This statement is incorrect. The reduction potential value is negative, which means that the element A has a strong tendency to undergo oxidation and lose electrons, rather than undergoing reduction and gaining electrons.
d) A will undergo oxidation easily - This statement is correct. The reduction potential value of element A is negative, which means that the element A has a strong tendency to undergo oxidation and lose electrons. The more negative the reduction potential value, the easier it is for the element to undergo oxidation and lose electrons. Therefore, element A will undergo oxidation easily.
Conclusion
The correct answer is option 'D'. The reduction potential value of an element indicates its tendency to undergo oxidation or reduction. A negative reduction potential value indicates a strong tendency to undergo oxidation and lose electrons, while a positive reduction potential value indicates a strong tendency to undergo reduction and gain electrons.
The reduction potential of an element A is -2.71V. This means that the element A has a strong tendency to undergo oxidation and lose electrons. The more negative the reduction potential value, the easier it is for the element to undergo oxidation and lose electrons.
Explanation of Options
a) A will be a good oxidising agent - This statement is incorrect. A good oxidizing agent is one that accepts electrons from other species and undergoes reduction. But, since the reduction potential of element A is negative, it indicates that the element A is likely to undergo oxidation and lose electrons, so it is not a good oxidizing agent.
b) A will accept electrons easily - This statement is incorrect. The reduction potential value is negative, which means that the element A has a strong tendency to lose electrons and undergo oxidation, rather than accepting electrons and undergoing reduction.
c) A will undergo reduction easily - This statement is incorrect. The reduction potential value is negative, which means that the element A has a strong tendency to undergo oxidation and lose electrons, rather than undergoing reduction and gaining electrons.
d) A will undergo oxidation easily - This statement is correct. The reduction potential value of element A is negative, which means that the element A has a strong tendency to undergo oxidation and lose electrons. The more negative the reduction potential value, the easier it is for the element to undergo oxidation and lose electrons. Therefore, element A will undergo oxidation easily.
Conclusion
The correct answer is option 'D'. The reduction potential value of an element indicates its tendency to undergo oxidation or reduction. A negative reduction potential value indicates a strong tendency to undergo oxidation and lose electrons, while a positive reduction potential value indicates a strong tendency to undergo reduction and gain electrons.
A half cell reaction A- → A + e- has a large negative reduction potential. It follows that :- a)A is easily reduced
- b)A – is easily reduced
- c)A – is easily oxidised
- d)A is easily oxidised
Correct answer is option 'C'. Can you explain this answer?
A half cell reaction A- → A + e- has a large negative reduction potential. It follows that :
a)
A is easily reduced
b)
A – is easily reduced
c)
A – is easily oxidised
d)
A is easily oxidised
|
|
Kalyan Chavan answered |
Can you please provide more details or context about the half-cell reaction you are referring to?
Hydrogen gas is not liberated when the following metal is added to dil. HCl.- a)Mg
- b)Sn
- c)Ag
- d)Zn
Correct answer is option 'C'. Can you explain this answer?
Hydrogen gas is not liberated when the following metal is added to dil. HCl.
a)
Mg
b)
Sn
c)
Ag
d)
Zn
|
|
Nikita Singh answered |
The metals, present below hydrogen in the electrochemical series, cannot liberate hydrogen from the dilute acids.
Among the given metals only Ag is present below hydrogen in electrochemical series, so it does not evolve hydrogen with dil HCl.
Ag−I−dilHCl ⟶ No reaction
Among the given metals only Ag is present below hydrogen in electrochemical series, so it does not evolve hydrogen with dil HCl.
Ag−I−dilHCl ⟶ No reaction
Can you explain the answer of this question below:Nernst equation for an electrode is based on the variation of electrode potential of an electrode with:
- A:
temperature only
- B:
Concentration of electrolyte only
- C:
Both a and b
- D:
Density of the electrodes
The answer is c.
Nernst equation for an electrode is based on the variation of electrode potential of an electrode with:
temperature only
Concentration of electrolyte only
Both a and b
Density of the electrodes
|
|
Nikita Singh answered |
Nernst equation for an electrode is based on the variation of electrode potential of an electrode with temperature and concentration of electrolyte.
Temperature for the measurement of standard electrode potential is- a)298K
- b)300K
- c)30?C
- d)310K
Correct answer is option 'A'. Can you explain this answer?
Temperature for the measurement of standard electrode potential is
a)
298K
b)
300K
c)
30?C
d)
310K
|
|
Anaya Patel answered |
The standard electrode potentials are customarily determined at solute concentrations of 1 Molar, gas pressures of 1 atmosphere, and a standard temperature which is usually 25°C i.e, 298 K.
Cell reaction is spontaneous when- a)EθRed is positive
- b)ΔGθ is positive
- c)ΔGθ is negative
- d)EθRed is negative.
Correct answer is option 'C'. Can you explain this answer?
Cell reaction is spontaneous when
a)
EθRed is positive
b)
ΔGθ is positive
c)
ΔGθ is negative
d)
EθRed is negative.
|
|
Nandini Patel answered |
ΔGdegree must be negative for the reaction to be spontaneous.
Calculate the standard cell potentials of galvanic cell, ∆rG and equilibrium constant of the reactions if the reaction is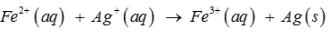
- a)0.01V, – 2.800 kJ mol–1, 3.2
- b)0.03V, – 2.895 kJ mol–1, 3.2
- c)0.02V, – 2.850 kJ mol–1, 3.2
- d)0.04V, – 2.955 kJ mol–1, 3.2
Correct answer is option 'B'. Can you explain this answer?
Calculate the standard cell potentials of galvanic cell, ∆rG and equilibrium constant of the reactions if the reaction is
a)
0.01V, – 2.800 kJ mol–1, 3.2
b)
0.03V, – 2.895 kJ mol–1, 3.2
c)
0.02V, – 2.850 kJ mol–1, 3.2
d)
0.04V, – 2.955 kJ mol–1, 3.2

|
Infinity Academy answered |
The standard emf of a galvanic cell involving 3 moles of electrons in its redox reaction is 0.59V.
Eºcell = 0.59V
n=3
The equilibrium constant for the reaction of the cell is given by the expression: ln K = RT nFEºcell
ln K = 8.314 × 298 × 3 × 96500 × 0.59 = 68.9
K≈1030
n=3
The equilibrium constant for the reaction of the cell is given by the expression: ln K = RT nFEºcell
ln K = 8.314 × 298 × 3 × 96500 × 0.59 = 68.9
K≈1030
The equilibrium constant for the reaction of the cell is 1030.
Salt bridge is indicated in the cell representation by :- a)I
- b)!!
- c)((
- d)II
Correct answer is option 'D'. Can you explain this answer?
Salt bridge is indicated in the cell representation by :
a)
I
b)
!!
c)
((
d)
II
|
|
Khushi Pandey answered |
Indicàted by Twø parallel linés (||)
. In the construction of a salt bridge, saturated solution of KNO3 is used because:- a)Velocity of K+ and NO3– are same
- b)Velocity of NO3– is greater than that of K+
- c)Velocity of K+ is greater than that of NO3–
- d)KNO3 is highly soluble in water
Correct answer is option 'A'. Can you explain this answer?
. In the construction of a salt bridge, saturated solution of KNO3 is used because:
a)
Velocity of K+ and NO3– are same
b)
Velocity of NO3– is greater than that of K+
c)
Velocity of K+ is greater than that of NO3–
d)
KNO3 is highly soluble in water
|
|
Riya Agarwal answered |
Velocities of both should be same to balance the amount of both ions in the soln. if the vel of any of them is more...then its ions will release more
The free energy change for the following cell reaction is given as :
2Au3+ (aq) + 3Cu (s) → 2Au (s) + 3Cu2+ (aq)
- a)6 FE°cell
- b)3 FE°cell
- c)-2 FE°cell
- d)-6 FE°cell
Correct answer is option 'D'. Can you explain this answer?
The free energy change for the following cell reaction is given as :
2Au3+ (aq) + 3Cu (s) → 2Au (s) + 3Cu2+ (aq)
2Au3+ (aq) + 3Cu (s) → 2Au (s) + 3Cu2+ (aq)
a)
6 FE°cell
b)
3 FE°cell
c)
-2 FE°cell
d)
-6 FE°cell
|
|
Preeti Iyer answered |
The correct answer is Option D.
EO = EOCa2+/ Ca - EOAu2+/ Au
= -2.87 - (1.50)
= -2.87 - 1.50
= -4.37 V
rGO = -nFEO
= -6 FEO
= -2.87 - (1.50)
= -2.87 - 1.50
= -4.37 V
rGO = -nFEO
= -6 FEO
Select the correct statement(s).- a)
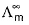 (molar conductance at infinite dilution) of strong electrolytes as well as weak electrolytes can be obtained by Kohlrausch’s law.
(molar conductance at infinite dilution) of strong electrolytes as well as weak electrolytes can be obtained by Kohlrausch’s law. 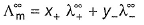
- b)
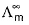 of strong as well as weak electrolytes can be obtained by extrapolation of graph between
of strong as well as weak electrolytes can be obtained by extrapolation of graph between 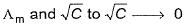
- c)
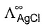 can be obtained by known value of
can be obtained by known value of 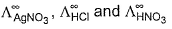
- d)
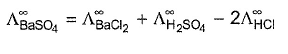
Correct answer is option 'A,C,D'. Can you explain this answer?
Select the correct statement(s).
a)
b)
c)
d)
|
|
Anaya Patel answered |
For strong electrolyte, variation in graph is uniform, hence
In case of weak electrolyte, variation of
Thus, (b) is incorrect.
Thus, correct based on Kohlrausch’s law.
Thus, it is also correct based on Kohlrausch's law.
Comprehension TypeDirection : This section contains 2 paragraphs, each describing theory, experiments, data, etc. Four questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IConsider the following solutions of an electrolyte
 Q. Conductivity.of 0.2 M solution is
Q. Conductivity.of 0.2 M solution is- a)2.60 Sm-1
- b)0.25 Sm-1
- c)4.00 Sm-1
- d)0.50 Sm-1
Correct answer is option 'B'. Can you explain this answer?
Comprehension Type
Direction : This section contains 2 paragraphs, each describing theory, experiments, data, etc. Four questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
Consider the following solutions of an electrolyte

Q.
Conductivity.of 0.2 M solution is
a)
2.60 Sm-1
b)
0.25 Sm-1
c)
4.00 Sm-1
d)
0.50 Sm-1
|
|
Krishna Iyer answered |
For 0.1 M solution, k = 1.30 Sm-1
Conductivity (k) = Conductance x Cell constant
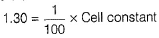
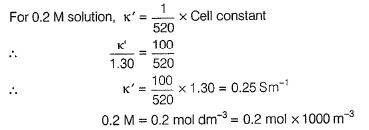

Conductivity (k) = Conductance x Cell constant
Consider the following reaction which of the following statement is true for this cell reaction.
(Zn + Cu2+ → Zn2+ + Cu)- a)Zn2+ ions are oxidized to Zn
- b)Zn is oxidized to Zn2+ ions
- c)Zn is reduced to Zn2+ ions
- d)Cu2+ ions are oxidized to Cu
-
Correct answer is option 'B'. Can you explain this answer?
Consider the following reaction which of the following statement is true for this cell reaction.
(Zn + Cu2+ → Zn2+ + Cu)
(Zn + Cu2+ → Zn2+ + Cu)
a)
Zn2+ ions are oxidized to Zn
b)
Zn is oxidized to Zn2+ ions
c)
Zn is reduced to Zn2+ ions
d)
Cu2+ ions are oxidized to Cu
|
|
Geetika Shah answered |
- For the reaction Zn + Cu2+ → Zn2+ + Cu, Zn is oxidized to Zn2+ while Cu2+ is reduced to Cu
- In a redox reaction, the reactant that loses electrons (is oxidized) causes a reduction and is called a reducing agent. In the example above, zinc metal is the reducing agent; it loses two electrons (is oxidized) and becomes Zn2+ ion.
The reduction potential of an element A is -2.71V.What can be concluded from this?- a)A will be a good oxidising agent
- b)A will accept electrons easily
- c)A will undergo reduction easily
- d)A will undergo oxidation easily
Correct answer is option 'D'. Can you explain this answer?
The reduction potential of an element A is -2.71V.What can be concluded from this?
a)
A will be a good oxidising agent
b)
A will accept electrons easily
c)
A will undergo reduction easily
d)
A will undergo oxidation easily

|
Dr Manju Sen answered |
Reduction potential means to accept electrons to reduce oneself.
A + e- → A- ∆Ereduction = +ve value
Since, the reduction potential is negative, it means that the reaction will reverse to make ∆E value +ve. So the reaction becomes,
A → A+ + e-
This becomes oxidation of A. So oxidation of A will be easy.
A + e- → A- ∆Ereduction = +ve value
Since, the reduction potential is negative, it means that the reaction will reverse to make ∆E value +ve. So the reaction becomes,
A → A+ + e-
This becomes oxidation of A. So oxidation of A will be easy.
The reduction potential of an element A is 1.71 V. What can be concluded from this?- a)A will lose electrons easily
- b)A will undergo reduction easily
- c)A will undergo oxidation easily
- d)A will be a good reducing agent
Correct answer is option 'B'. Can you explain this answer?
The reduction potential of an element A is 1.71 V. What can be concluded from this?
a)
A will lose electrons easily
b)
A will undergo reduction easily
c)
A will undergo oxidation easily
d)
A will be a good reducing agent
|
|
Rajeev Saxena answered |
The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The more positive the potential is the more likely it will be reduced. Hence, A will undergo reduction easily.
In an electrolytic cell current flows from -- a)Cathode to anode in outer circuit
- b)Anode to cathode outside the cell
- c)Cathode to anode inside the cell
- d)Anode to cathode inside the cell
Correct answer is option 'A'. Can you explain this answer?
In an electrolytic cell current flows from -
a)
Cathode to anode in outer circuit
b)
Anode to cathode outside the cell
c)
Cathode to anode inside the cell
d)
Anode to cathode inside the cell
|
|
Krishna Iyer answered |
In an electrolytic cell, current flows from cathode to anode in outer circuit and in daniell cell, it is the reverse direction of flow of current from anode to cathode in outer circuit.
Resistance of 0.2 M soluton of an electrolyte is 50?. The specific conductance of solution is 1.3 Sm-1. If resistance of the 0.4 M solution of the same electrolyte is 260Ω, its molar conductivity is[AlEEE 2011]- a)6250 S m2 mol-1
- b)6.25 x 10-4 S m2 mol-1
- c)62.5 x 10-4 S m2 mol-1
- d)62.5 S m2 mol-1
Correct answer is option 'B'. Can you explain this answer?
Resistance of 0.2 M soluton of an electrolyte is 50?. The specific conductance of solution is 1.3 Sm-1. If resistance of the 0.4 M solution of the same electrolyte is 260Ω, its molar conductivity is
[AlEEE 2011]
a)
6250 S m2 mol-1
b)
6.25 x 10-4 S m2 mol-1
c)
62.5 x 10-4 S m2 mol-1
d)
62.5 S m2 mol-1
|
|
Gaurav Kumar answered |
Specific conductance (k) of 0.2 M solution
= Conductance x Cell constant
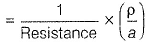
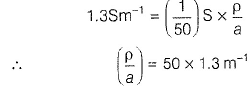
Specific conductance of 0.4M solution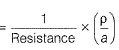
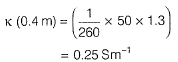

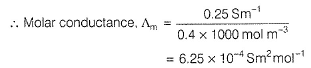
= Conductance x Cell constant
Specific conductance of 0.4M solution
Stronger the oxidizing agent, greater is the:- a)Reactivity
- b)Ionic behaviour
- c)Oxidation potential
- d)Reduction potential
Correct answer is option 'D'. Can you explain this answer?
Stronger the oxidizing agent, greater is the:
a)
Reactivity
b)
Ionic behaviour
c)
Oxidation potential
d)
Reduction potential
|
|
Rajeev Saxena answered |
Lithium is strongest Reducing agent because of lowest standard reduction potential. When something is oxidized, it reduces another substance, becoming a reducing agent. Hence lithium is the strongest reducing agent. remember, Li is the strongest reducing agent and F is the strongest oxidizing agent!
At 300K molar conductivity of solution A is 350 units, and at infinite dilution the molar conductivity of the same sample is 480 unit. Predict the percentage dissociation of the electrolyte.- a)73.0%
- b)37.0%
- c)63.0%
- d)137.0%
Correct answer is option 'A'. Can you explain this answer?
At 300K molar conductivity of solution A is 350 units, and at infinite dilution the molar conductivity of the same sample is 480 unit. Predict the percentage dissociation of the electrolyte.
a)
73.0%
b)
37.0%
c)
63.0%
d)
137.0%

|
Gunjan Lakhani answered |
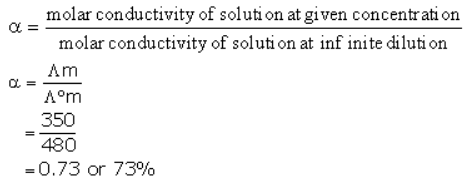
Which of the following solutions has the highest equivalent conductance ?- a)0.01M NaCl
- b)0.050 M NaCl
- c)0.005M NaCl
- d)0.02M NaCl
Correct answer is option 'C'. Can you explain this answer?
Which of the following solutions has the highest equivalent conductance ?
a)
0.01M NaCl
b)
0.050 M NaCl
c)
0.005M NaCl
d)
0.02M NaCl
|
|
Preeti Iyer answered |
Higher the dilution higher will be the equivalent conductance
EMF of the following cell is 0.2905 V 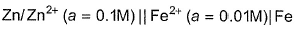 The equilibrium constant for the cell reaction is [IIT JEE 2004]
The equilibrium constant for the cell reaction is [IIT JEE 2004]- a)100.32/0.059
- b)100.32/0.0295
- c)100.26/0.0295
- d)100.32/0.295
Correct answer is option 'B'. Can you explain this answer?
EMF of the following cell is 0.2905 V
The equilibrium constant for the cell reaction is
[IIT JEE 2004]
a)
100.32/0.059
b)
100.32/0.0295
c)
100.26/0.0295
d)
100.32/0.295

|
Dilip Chaurasiya answered |
E0 cell = .0591/n log k. k= equilibrium constant
On the basis of the following E° values, the strongest oxidizing agent is: 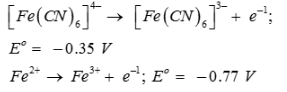
- a)[Fe(CN)6]4-
- b)Fe2+
- c)Fe3+
- d)[Fe(CN)6]3-
Correct answer is option 'C'. Can you explain this answer?
On the basis of the following E° values, the strongest oxidizing agent is:
a)
[Fe(CN)6]4-
b)
Fe2+
c)
Fe3+
d)
[Fe(CN)6]3-

|
Dishani Kulkarni answered |
Strongest oxidizing agent is one having more positive or less negative reduction potential.
For the cell, 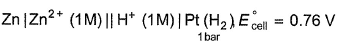 and for the cell Pt(H2) | H+ (1M)| Ag,
and for the cell Pt(H2) | H+ (1M)| Ag, 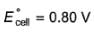 Thus Ecell for theAg|Ag+ (0.1M) || Zn2+ (0.1M) | Zn is ....................and cell reaction is...............
Thus Ecell for theAg|Ag+ (0.1M) || Zn2+ (0.1M) | Zn is ....................and cell reaction is...............- a)1.44 V ,spontaneous
- b)0.4 V ,spontaneous
- c)-1.44 V ,non-spontaneous
- d)-1.53 V ,non-spontaneous
Correct answer is option 'D'. Can you explain this answer?
For the cell, 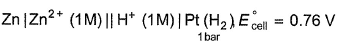 and for the cell Pt(H2) | H+ (1M)| Ag,
and for the cell Pt(H2) | H+ (1M)| Ag, 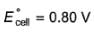
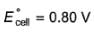
Thus Ecell for the
Ag|Ag+ (0.1M) || Zn2+ (0.1M) | Zn is ....................and cell reaction is...............
a)
1.44 V ,spontaneous
b)
0.4 V ,spontaneous
c)
-1.44 V ,non-spontaneous
d)
-1.53 V ,non-spontaneous
|
|
Geetika Shah answered |
Ecell < 0, hence reaction is non-spontaneous.
Kohlrausch’s Law shows that:- a)at infinite dilution the ionic conductivity of ions is additive.
- b)at infinite dilution the ionic conductivity of all the ions of the electrolyte become equal.
- c)at infinite dilution the concentration of the electrolyte becomes unity.
- d)at infinite dilution the concentration of ions increases.
Correct answer is option 'A'. Can you explain this answer?
Kohlrausch’s Law shows that:
a)
at infinite dilution the ionic conductivity of ions is additive.
b)
at infinite dilution the ionic conductivity of all the ions of the electrolyte become equal.
c)
at infinite dilution the concentration of the electrolyte becomes unity.
d)
at infinite dilution the concentration of ions increases.
|
|
Preeti Khanna answered |
The correct answer is option A
Kohlrausch's law states that the equivalent conductivity of an electrolyte at infinite dilution is equal to the sum of the conductances of the anions and cations. If a salt is dissolved in water, the conductivity of the solution is the sum of the conductances of the anions and cations.
Hence, at infinite dilution the ionic conductivity of ions is additive.
Hence, at infinite dilution the ionic conductivity of ions is additive.
In the equation, ΔG° = – nF E° cell ; F is:- a)Boltzmann constant
- b)Faraday’s constant
- c)Gas constant
- d)Universal gas constant
Correct answer is option 'B'. Can you explain this answer?
In the equation, ΔG° = – nF E° cell ; F is:
a)
Boltzmann constant
b)
Faraday’s constant
c)
Gas constant
d)
Universal gas constant
|
|
Nandini Iyer answered |
The correct answer is Option B.
The relationship between ΔGo and Eo is given by the following equation: ΔGo=−nFEo. Here, n is the number of moles of electrons and F is the Faraday constant.
The relationship between ΔGo and Eo is given by the following equation: ΔGo=−nFEo. Here, n is the number of moles of electrons and F is the Faraday constant.
The electrode potential measures the :- a)tendency of a cell reaction to occur
- b)current carried by an elelctrode
- c)tendency of the electrode to gain or lose electrons
- d)difference in the ionisation of electrode and metal ion
Correct answer is option 'C'. Can you explain this answer?
The electrode potential measures the :
a)
tendency of a cell reaction to occur
b)
current carried by an elelctrode
c)
tendency of the electrode to gain or lose electrons
d)
difference in the ionisation of electrode and metal ion
|
|
Nandini Patel answered |
The tendency of an electrode to lose or gain electrons when it is in contact with its own ions in solution is called electrode potential.
Since the tendency to lose electrons means also the tendency to get oxidised, this tendency is called oxidation potential. Similarly, the tendency to gain electrons means the tendency to get reduced. Hence this tendency is called reduction potential.
Correct arrangement of Al, Cu, Fe, Mg and Zn in the order in which they displace each other from the solution of their salts is- a)Mg, Al, Zn, Fe, Cu
- b)Mg, Al, Zn, Cu, Fe
- c)Mg, Al, Cu, Fe, Zn
- d)Cu, Al, Zn, Fe, Mg
Correct answer is option 'A'. Can you explain this answer?
Correct arrangement of Al, Cu, Fe, Mg and Zn in the order in which they displace each other from the solution of their salts is
a)
Mg, Al, Zn, Fe, Cu
b)
Mg, Al, Zn, Cu, Fe
c)
Mg, Al, Cu, Fe, Zn
d)
Cu, Al, Zn, Fe, Mg

|
Mehul Choudhary answered |
Reactivity series.
Consider the cell reaction:
Cd(s) | Cd2+ (1.0 M) || Cu2+ (1.0 m) | Cu (s)
If we wish to make a cell with more positive voltage using the same substances, we should:
- a)Increase [Cd2+] as well as [Cu2+] to 2.0 M
- b)Increase only [Cu2+] to 2.0 M
- c)Reduce only [Cd2+] to 0.1 M
- d)Decreases [Cd2+] to 0.1M and increases [Cu2+] to 1.0M
Correct answer is option 'D'. Can you explain this answer?
Consider the cell reaction:
Cd(s) | Cd2+ (1.0 M) || Cu2+ (1.0 m) | Cu (s)
If we wish to make a cell with more positive voltage using the same substances, we should:
Cd(s) | Cd2+ (1.0 M) || Cu2+ (1.0 m) | Cu (s)
If we wish to make a cell with more positive voltage using the same substances, we should:
a)
Increase [Cd2+] as well as [Cu2+] to 2.0 M
b)
Increase only [Cu2+] to 2.0 M
c)
Reduce only [Cd2+] to 0.1 M
d)
Decreases [Cd2+] to 0.1M and increases [Cu2+] to 1.0M
|
|
Geetika Shah answered |
The correct answer is Option D.
Redox reaction:
Cd(s)→Cd2++2e
Cu2++2e→Cu(s)
Ecell = E°cell − (0.059/2) log ([Cd2+]/ [Cu2+])
Decreases [Cd2+] to 0.1M and increases [Cu2+] to 1.0M
Cd(s)→Cd2++2e
Cu2++2e→Cu(s)
Ecell = E°cell − (0.059/2) log ([Cd2+]/ [Cu2+])
Decreases [Cd2+] to 0.1M and increases [Cu2+] to 1.0M
In an electrochemical cell, the electrode having a lower reduction potential will act as:- a)Salt bridge
- b)Electrolyte
- c)Anode
- d)Cathode
Correct answer is option 'C'. Can you explain this answer?
In an electrochemical cell, the electrode having a lower reduction potential will act as:
a)
Salt bridge
b)
Electrolyte
c)
Anode
d)
Cathode
|
|
Sargam Singh answered |
A substance with lower reduction potential has more tendency to oxidize .in a electrochemical cell anode performs oxidation reaction hence the electrode will function as a anode
During electrolysis, the reaction that takes place at cathode is:- a)Hydrolysis
- b)Reduction
- c)Oxidation
- d)Neutralization
Correct answer is option 'B'. Can you explain this answer?
During electrolysis, the reaction that takes place at cathode is:
a)
Hydrolysis
b)
Reduction
c)
Oxidation
d)
Neutralization
|
|
Rajat Patel answered |
The electrode at which oxidation takes place is known as the anode, while the electrode at which reduction take place is called the cathode. If you see galvanic cell reduction take place at the left electrode, so the left one is the cathode. Oxidation takes place at the right electrode, so the right one is the anode.
Gibbs free energy change for a cell reaction is positive what does it indicates?- a)cell will discharge easily
- b)Cell reaction is spontaneous
- c)Cell reaction is non spontaneous
- d)Cell will work under standard conditions
Correct answer is option 'C'. Can you explain this answer?
Gibbs free energy change for a cell reaction is positive what does it indicates?
a)
cell will discharge easily
b)
Cell reaction is spontaneous
c)
Cell reaction is non spontaneous
d)
Cell will work under standard conditions
|
|
Rajeev Saxena answered |
No, reaction cannot be spontaneous (continue to happen) when the change in gibbs free energy is positive. ... For a spontaneous process to happen , the change in Gibbs free energy must be negative. A roaring bonfire is an example of a spontaneous reaction.
The variation of equivalent conductance vs decrease in concentration of a strong electrolyte is correctly given in the plot -- a) A
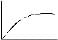
- b) A
- c)A

- d) A

Correct answer is option 'A'. Can you explain this answer?
The variation of equivalent conductance vs decrease in concentration of a strong electrolyte is correctly given in the plot -
a)
A
b)
A
c)
A
d)
A
|
|
Om Desai answered |
On decreasing the value of M will increase but increase will be hyberbolic.
One of the simplest methods of preventing corrosion is to prevent the surface of the metallic object to come in contact with atmosphere. This can be done- a)by covering the surface with oil
- b)by covering the surface with salt
- c)by covering the surface with citric acid
- d)by covering the surface with paint or by some chemicals
Correct answer is option 'D'. Can you explain this answer?
One of the simplest methods of preventing corrosion is to prevent the surface of the metallic object to come in contact with atmosphere. This can be done
a)
by covering the surface with oil
b)
by covering the surface with salt
c)
by covering the surface with citric acid
d)
by covering the surface with paint or by some chemicals
|
|
Rajesh Chatterjee answered |
Preventing corrosion is essential to ensure the longevity and integrity of metallic objects. One of the simplest and most effective methods to prevent corrosion is by covering the surface of the object with paint or certain chemicals. This method creates a protective barrier between the metal surface and the atmosphere, preventing contact and thereby reducing the chances of corrosion.
Here is a detailed explanation of why covering the surface with paint or chemicals is an effective method of preventing corrosion:
1. Creation of a Barrier: By covering the surface of the metallic object with paint or chemicals, a physical barrier is created between the metal and the surrounding atmosphere. This barrier prevents the metal from coming into direct contact with oxygen, moisture, and other corrosive elements present in the air.
2. Protection from Moisture: Moisture is one of the primary causes of corrosion. When metal comes into contact with moisture, it undergoes a chemical reaction known as oxidation, leading to the formation of rust. By covering the surface, the paint or chemicals act as a waterproof layer, preventing moisture from reaching the metal surface and reducing the chances of corrosion.
3. Prevention of Oxygen Exposure: Oxygen is another key component in the corrosion process. By covering the surface, the paint or chemicals act as a barrier, preventing oxygen from reaching the metal surface. This helps in inhibiting the oxidation reaction and reducing the chances of corrosion.
4. Chemical Protection: Some paints and chemicals used for covering the surface of metallic objects contain corrosion inhibitors. These inhibitors are chemicals that actively work to prevent or slow down the corrosion process. They form a protective layer on the metal surface, hindering the reaction between the metal and corrosive elements.
5. Flexibility and Ease of Application: Paints and chemicals offer the advantage of being flexible and easy to apply on different types of metal surfaces. They can be brushed, sprayed, or dipped, allowing for complete coverage and protection against corrosion.
It is important to note that the choice of paint or chemical coating depends on the specific requirements of the metal object, the environment it will be exposed to, and the type of corrosion it is susceptible to. Consulting with experts or conducting thorough research is recommended to ensure the most suitable protective coating is applied.
Here is a detailed explanation of why covering the surface with paint or chemicals is an effective method of preventing corrosion:
1. Creation of a Barrier: By covering the surface of the metallic object with paint or chemicals, a physical barrier is created between the metal and the surrounding atmosphere. This barrier prevents the metal from coming into direct contact with oxygen, moisture, and other corrosive elements present in the air.
2. Protection from Moisture: Moisture is one of the primary causes of corrosion. When metal comes into contact with moisture, it undergoes a chemical reaction known as oxidation, leading to the formation of rust. By covering the surface, the paint or chemicals act as a waterproof layer, preventing moisture from reaching the metal surface and reducing the chances of corrosion.
3. Prevention of Oxygen Exposure: Oxygen is another key component in the corrosion process. By covering the surface, the paint or chemicals act as a barrier, preventing oxygen from reaching the metal surface. This helps in inhibiting the oxidation reaction and reducing the chances of corrosion.
4. Chemical Protection: Some paints and chemicals used for covering the surface of metallic objects contain corrosion inhibitors. These inhibitors are chemicals that actively work to prevent or slow down the corrosion process. They form a protective layer on the metal surface, hindering the reaction between the metal and corrosive elements.
5. Flexibility and Ease of Application: Paints and chemicals offer the advantage of being flexible and easy to apply on different types of metal surfaces. They can be brushed, sprayed, or dipped, allowing for complete coverage and protection against corrosion.
It is important to note that the choice of paint or chemical coating depends on the specific requirements of the metal object, the environment it will be exposed to, and the type of corrosion it is susceptible to. Consulting with experts or conducting thorough research is recommended to ensure the most suitable protective coating is applied.
Three cell A, B and C has equilibrium constant in the ratio 1:4 : 9 respectively. Arrange the following cells in the order of increasing Gibbs free energy.- a)A>B>C
- b)Cannot be answered
- c)B>C>A
- d)A
Correct answer is option 'A'. Can you explain this answer?
Three cell A, B and C has equilibrium constant in the ratio 1:4 : 9 respectively. Arrange the following cells in the order of increasing Gibbs free energy.
a)
A>B>C
b)
Cannot be answered
c)
B>C>A
d)
A
|
|
Nandini Patel answered |
A>B>C
Smaller the value of equilibrium constant (k) larger will be value of Gibbs free energy.
During electrolysis, the reaction that takes place at anode is:- a)Reduction
- b)Neutralization
- c)Hydrolysis
- d)Oxidation
Correct answer is option 'D'. Can you explain this answer?
During electrolysis, the reaction that takes place at anode is:
a)
Reduction
b)
Neutralization
c)
Hydrolysis
d)
Oxidation
|
|
Rajat Patel answered |
Oxidation takes place at the right electrode, so the right one is the anode. While in electrolytic cell reduction takes place at the right electrode, so right one is the cathode. Oxidation takes place at the left electrode, so the left one is the anode.
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1.- a)0.219 cm-1
- b)0.239 cm-1
- c)0.229 cm-1
- d)0.209 cm-1
Correct answer is option 'A'. Can you explain this answer?
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1.
a)
0.219 cm-1
b)
0.239 cm-1
c)
0.229 cm-1
d)
0.209 cm-1
|
|
Preeti Iyer answered |
Κ = G x cell constant and G = 1/R.
The standard electrode potential is measured by- a)Galvanometer
- b)Voltmeter
- c)Electrometer
- d)Pyrometer
Correct answer is option 'B'. Can you explain this answer?
The standard electrode potential is measured by
a)
Galvanometer
b)
Voltmeter
c)
Electrometer
d)
Pyrometer

|
Gauri Khanna answered |
Voltmeter measures the potential.
For the following cell with hydrogen electrodes at two different pressure p1 and p2 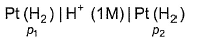 emf is given by
emf is given by- a)
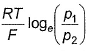
- b)
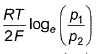
- c)
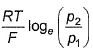
- d)
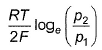
Correct answer is option 'B'. Can you explain this answer?
For the following cell with hydrogen electrodes at two different pressure p1 and p2 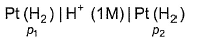
emf is given by
a)
b)
c)
d)
|
|
Krishna Iyer answered |
For SHE E°SHE = 0.00 V
Oxidation at anode (left)
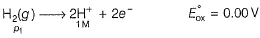
Reduction at cathode (right)
Net
Oxidation at anode (left)
Reduction at cathode (right)
Net
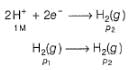
This is the type of the cell in which electrodes at different pressures are dipped in same electrolyte and connectivity is made by a salt-bridge.
Reaction Quotient (Q) 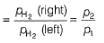
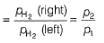
∵
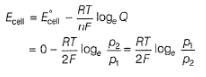
At equilibrium:- a)Cell potential’ E cell‘ becomes zero
- b)Equilibrium constant becomes equal to electrode potential
- c)Equilibrium constant becomes zero
- d)Cell potential ‘E cell‘ becomes unity
Correct answer is option 'A'. Can you explain this answer?
At equilibrium:
a)
Cell potential’ E cell‘ becomes zero
b)
Equilibrium constant becomes equal to electrode potential
c)
Equilibrium constant becomes zero
d)
Cell potential ‘E cell‘ becomes unity

|
Ayush Joshi answered |
E cell is 0 in equilibrium that is E cathode becomes equal to E anode ………. ... So E cell is zero at equilibrium that is when the E(cathode) becomes equal to E(anode). E deg cell is zero in the concentration cell when both the electrodes are of the same metal.
One mole of electron passes through each of the solution of AgNO3, CuSO4 and AlCl3 when Ag, Cu and Al are deposited at cathode. The molar ratio of Ag, Cu and Al deposited are- a)1 : 1 : 1
- b)6 : 3 : 2
- c)6 : 3 : 1
- d)1 : 3 : 6
Correct answer is option 'B'. Can you explain this answer?
One mole of electron passes through each of the solution of AgNO3, CuSO4 and AlCl3 when Ag, Cu and Al are deposited at cathode. The molar ratio of Ag, Cu and Al deposited are
a)
1 : 1 : 1
b)
6 : 3 : 2
c)
6 : 3 : 1
d)
1 : 3 : 6
|
|
Anisha Bose answered |
Molar ratio
All have the same equivalent
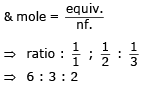
All have the same equivalent
A solution of Fe2+ is titrated potentiometrically using Ce4+ solution.Fe2+ → Fe3+ + e- , E0 = -0.77 Vemf of the Pt | Fe2+ , Fe3+ pair at 50% and 90% titration of Fe2+ are - a)0.77 V , 0.826 V
- b)-0.826 V , -0.77 V
- c)-0.77 V ,-0.826 V
- d)0.00 V , 0.00 V
Correct answer is option 'C'. Can you explain this answer?
A solution of Fe2+ is titrated potentiometrically using Ce4+ solution.
Fe2+ → Fe3+ + e- , E0 = -0.77 V
emf of the Pt | Fe2+ , Fe3+ pair at 50% and 90% titration of Fe2+ are
a)
0.77 V , 0.826 V
b)
-0.826 V , -0.77 V
c)
-0.77 V ,-0.826 V
d)
0.00 V , 0.00 V
|
|
Lavanya Menon answered |
When Fe2+ is 50% titrated
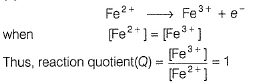
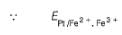 =
=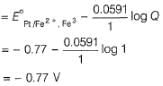
where Fe2+ is 90% titrated
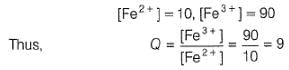
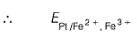
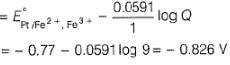
The cell representation of the given reaction is:
Zn(s) + Cu2+ → Zn2+ + Cu(s)- a)Zn|zn2+||Cu2+|Cu
- b)Cu2+|Cu||Zn|zn2+
- c)Zn|zn2+||Cu|Cu2+
- d)Cu|Cu2+||zn2+|Zn
Correct answer is option 'A'. Can you explain this answer?
The cell representation of the given reaction is:
Zn(s) + Cu2+ → Zn2+ + Cu(s)
Zn(s) + Cu2+ → Zn2+ + Cu(s)
a)
Zn|zn2+||Cu2+|Cu
b)
Cu2+|Cu||Zn|zn2+
c)
Zn|zn2+||Cu|Cu2+
d)
Cu|Cu2+||zn2+|Zn
|
|
Atishay Jain answered |
Answer is A because Zn oxidized in Zn+2 and Cu+2 reduced in Cu.So Zn is anode and Cu is cathode.
A conductance cell was filled with a 0.02 M KCl solution which has a specific conductance of 2.768 × 10-3 ohm-1 cm-1. If its resistance is 82.4 ohm at 25ºC, the cell constant is-- a)0.2182 cm-1
- b)0.2281 cm-1
- c)0.2821 cm-1
- d)0.2381 cm-1
Correct answer is option 'B'. Can you explain this answer?
A conductance cell was filled with a 0.02 M KCl solution which has a specific conductance of 2.768 × 10-3 ohm-1 cm-1. If its resistance is 82.4 ohm at 25ºC, the cell constant is-
a)
0.2182 cm-1
b)
0.2281 cm-1
c)
0.2821 cm-1
d)
0.2381 cm-1
|
|
Vivek Rana answered |
K = G. L/A
10–3 × 2.768 = 1/R ×L/A
L/A = 228.08 × 10–3
= 0.2281 cm–1
10–3 × 2.768 = 1/R ×L/A
L/A = 228.08 × 10–3
= 0.2281 cm–1
Chapter doubts & questions for Electrochemistry - Chemistry CUET UG Mock Test Series 2026 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Electrochemistry - Chemistry CUET UG Mock Test Series 2026 in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup