All Exams >
Class 9 >
Science Olympiad Class 9 >
All Questions
All questions of Matter in Our Surroundings for Class 9 Exam
Which of the following process/ processes release heat?(i) Condensation (ii) Vaporisation(iii) Freezing (iv) Melting- a)(i) and (ii)
- b)(i) and (iii)
- c)only (ii)
- d)Only (iv)
Correct answer is option 'B'. Can you explain this answer?
Which of the following process/ processes release heat?
(i) Condensation (ii) Vaporisation
(iii) Freezing (iv) Melting
a)
(i) and (ii)
b)
(i) and (iii)
c)
only (ii)
d)
Only (iv)

|
Saranya Sengupta answered |
Condensation and Freezing Release Heat
- Condensation is the process by which a gas or vapor changes into a liquid state. When water vapor condenses, it releases heat energy to the surroundings. This occurs because the molecules in the gas phase have higher kinetic energy compared to the liquid phase. As they lose energy and slow down, they release heat. For example, when water vapor condenses on a cold surface to form dew, heat is released.
- Freezing is the process by which a liquid changes into a solid state. When a substance freezes, it releases heat energy. This is because the molecules in the liquid phase lose energy and slow down, releasing heat as they arrange themselves into a more ordered solid structure. For example, when water freezes into ice, heat is released.
Vaporization and Melting Absorb Heat
- Vaporization is the process by which a liquid changes into a gas or vapor. When a substance vaporizes, it absorbs heat energy from the surroundings. This occurs because the molecules in the liquid phase gain energy and speed up to overcome intermolecular forces and transition into the gas phase. For example, when water boils and turns into steam, heat is absorbed from the surroundings.
- Melting is the process by which a solid changes into a liquid state. When a substance melts, it absorbs heat energy. This is because the molecules in the solid phase gain energy and vibrate more rapidly, breaking the intermolecular forces holding them together and transitioning into the liquid phase. For example, when ice melts into water, heat is absorbed from the surroundings.
Conclusion
- The processes of condensation and freezing release heat, while vaporization and melting absorb heat.
- Condensation is the process by which a gas or vapor changes into a liquid state. When water vapor condenses, it releases heat energy to the surroundings. This occurs because the molecules in the gas phase have higher kinetic energy compared to the liquid phase. As they lose energy and slow down, they release heat. For example, when water vapor condenses on a cold surface to form dew, heat is released.
- Freezing is the process by which a liquid changes into a solid state. When a substance freezes, it releases heat energy. This is because the molecules in the liquid phase lose energy and slow down, releasing heat as they arrange themselves into a more ordered solid structure. For example, when water freezes into ice, heat is released.
Vaporization and Melting Absorb Heat
- Vaporization is the process by which a liquid changes into a gas or vapor. When a substance vaporizes, it absorbs heat energy from the surroundings. This occurs because the molecules in the liquid phase gain energy and speed up to overcome intermolecular forces and transition into the gas phase. For example, when water boils and turns into steam, heat is absorbed from the surroundings.
- Melting is the process by which a solid changes into a liquid state. When a substance melts, it absorbs heat energy. This is because the molecules in the solid phase gain energy and vibrate more rapidly, breaking the intermolecular forces holding them together and transitioning into the liquid phase. For example, when ice melts into water, heat is absorbed from the surroundings.
Conclusion
- The processes of condensation and freezing release heat, while vaporization and melting absorb heat.
On converting 0°C, –6°C, 273°C into Kelvin, the correct sequence of temperature will be- a)273 K, 267 K, 546 K
- b)273 K, 279 K, 546 K
- c)273 K, 267 K, 0 K
- d)–273 K, –279 K, 0 K
Correct answer is option 'A'. Can you explain this answer?
On converting 0°C, –6°C, 273°C into Kelvin, the correct sequence of temperature will be
a)
273 K, 267 K, 546 K
b)
273 K, 279 K, 546 K
c)
273 K, 267 K, 0 K
d)
–273 K, –279 K, 0 K
|
|
Shilpa Choudhury answered |
Temperature on Kelvin scale = Temperature
on Celsius scale + 273
(i) = 0 + 273
= 273 K
(ii) = – 6 + 273
= 267 K
(iii)= 273 + 273
= 546 K
On converting 308 K, 329 K and 391 K to Celsius scale, the correct sequence of temperature will be- a)33°C, 56°C and 118°C
- b)35°C, 56°C and 118°C
- c)35°C, 56°C and 119°C
- d)56°C, 119°C and 35°C
Correct answer is option 'B'. Can you explain this answer?
On converting 308 K, 329 K and 391 K to Celsius scale, the correct sequence of temperature will be
a)
33°C, 56°C and 118°C
b)
35°C, 56°C and 118°C
c)
35°C, 56°C and 119°C
d)
56°C, 119°C and 35°C
|
|
Sara Nair answered |
To convert temperatures from Kelvin (K) to Celsius (°C), we use the formula:
°C = K - 273.15
Let's apply this formula to the given temperatures:
1. For 308 K:
°C = 308 - 273.15 = 34.85 ≈ 35°C
2. For 329 K:
°C = 329 - 273.15 = 55.85 ≈ 56°C
3. For 391 K:
°C = 391 - 273.15 = 117.85 ≈ 118°C
Hence, the correct sequence of temperatures in Celsius scale is:
35°C, 56°C, and 118°C.
Explanation:
To convert temperatures from Kelvin to Celsius, we subtract 273.15 from the given temperature in Kelvin. This is because 0 K is the absolute zero temperature, which is equivalent to -273.15°C. So, to convert any temperature above absolute zero from Kelvin to Celsius, we need to subtract 273.15.
In the given question, we have three temperatures: 308 K, 329 K, and 391 K.
By applying the conversion formula, we find that 308 K is approximately equal to 35°C, 329 K is approximately equal to 56°C, and 391 K is approximately equal to 118°C.
Therefore, the correct sequence of temperatures in Celsius scale is 35°C, 56°C, and 118°C, which corresponds to option B.
°C = K - 273.15
Let's apply this formula to the given temperatures:
1. For 308 K:
°C = 308 - 273.15 = 34.85 ≈ 35°C
2. For 329 K:
°C = 329 - 273.15 = 55.85 ≈ 56°C
3. For 391 K:
°C = 391 - 273.15 = 117.85 ≈ 118°C
Hence, the correct sequence of temperatures in Celsius scale is:
35°C, 56°C, and 118°C.
Explanation:
To convert temperatures from Kelvin to Celsius, we subtract 273.15 from the given temperature in Kelvin. This is because 0 K is the absolute zero temperature, which is equivalent to -273.15°C. So, to convert any temperature above absolute zero from Kelvin to Celsius, we need to subtract 273.15.
In the given question, we have three temperatures: 308 K, 329 K, and 391 K.
By applying the conversion formula, we find that 308 K is approximately equal to 35°C, 329 K is approximately equal to 56°C, and 391 K is approximately equal to 118°C.
Therefore, the correct sequence of temperatures in Celsius scale is 35°C, 56°C, and 118°C, which corresponds to option B.
The boiling point of ethane is, –88°C. This temperature will be equivalent to- a)185 K
- b)361 K
- c)288 K
- d)285 K
Correct answer is option 'A'. Can you explain this answer?
The boiling point of ethane is, –88°C. This temperature will be equivalent to
a)
185 K
b)
361 K
c)
288 K
d)
285 K
|
|
Shilpa Choudhury answered |
Temperature on Kelvin scale = Temperature
on Celsius scale + 273
= – 88 + 273
= 185 k
Which one of the following statements is not true?- a)The molecules in a gas exert negligibly small forces on each other, except during collisions
- b)The molecules of a gas occupy all the space available
- c)The molecules in a liquid are arranged in a regular pattern
- d)The molecules in a solid vibrate about a fixed position
Correct answer is option 'C'. Can you explain this answer?
Which one of the following statements is not true?
a)
The molecules in a gas exert negligibly small forces on each other, except during collisions
b)
The molecules of a gas occupy all the space available
c)
The molecules in a liquid are arranged in a regular pattern
d)
The molecules in a solid vibrate about a fixed position
|
|
Shilpa Choudhury answered |
Molecules have arrangement
• In gas are well separated with no regular arrangement.
• In liquid are close together with no regular arrangement.
• In solid are tightly packed, usually in a regular pattern.
Which of the following are also considered to be the states of matter?(i) BEC (ii) BHC (iii) plasma (iv) platelets- a)(i) and (ii)
- b)(i) and (iii)
- c)(ii) and (iii)
- d)(iii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
Which of the following are also considered to be the states of matter?
(i) BEC (ii) BHC (iii) plasma (iv) platelets
a)
(i) and (ii)
b)
(i) and (iii)
c)
(ii) and (iii)
d)
(iii) and (iv)
|
|
Shilpa Choudhury answered |
Plasma and BEC (Bose-Einstein condensate) are also considered as states of matter because plasma is mixture of free atoms and ions and BEC occupies space and has mass.
When heat is constantly supplied by a burner to boiling water, then the temperature of water during vaporisation- a)Rises very slowly
- b)First rises and then becomes constant
- c)Rises rapidly until steam is produced
- d)Does not rise at all
Correct answer is option 'D'. Can you explain this answer?
When heat is constantly supplied by a burner to boiling water, then the temperature of water during vaporisation
a)
Rises very slowly
b)
First rises and then becomes constant
c)
Rises rapidly until steam is produced
d)
Does not rise at all
|
|
Yashasvi Reddy answered |
Answer:
When heat is constantly supplied by a burner to boiling water, the temperature of the water does not rise at all. This is because during the process of vaporization, the heat energy supplied is used to convert the liquid water into water vapor rather than increasing the temperature of the water.
Explanation:
When heat is supplied to boiling water, it undergoes the process of vaporization. Vaporization is the phase transition from a liquid to a gas, and it occurs at the boiling point of the substance. In the case of water, the boiling point is 100 degrees Celsius (212 degrees Fahrenheit) at atmospheric pressure.
During the process of vaporization, the heat energy supplied to the water is used to break the intermolecular forces between the water molecules, allowing them to escape into the surrounding air as water vapor. This process requires a large amount of energy, known as the latent heat of vaporization.
As the heat energy is absorbed by the water molecules and used for vaporization, the temperature of the water remains constant at the boiling point. This is because the energy is being used to change the state of the water rather than increase its temperature.
Once all the liquid water has been converted into water vapor, the temperature of the water can start to rise again as the heat energy supplied by the burner is no longer being used for vaporization.
Summary:
When heat is constantly supplied by a burner to boiling water, the temperature of the water does not rise at all. This is because the heat energy is used for the process of vaporization, converting the liquid water into water vapor, rather than increasing the temperature of the water.
When heat is constantly supplied by a burner to boiling water, the temperature of the water does not rise at all. This is because during the process of vaporization, the heat energy supplied is used to convert the liquid water into water vapor rather than increasing the temperature of the water.
Explanation:
When heat is supplied to boiling water, it undergoes the process of vaporization. Vaporization is the phase transition from a liquid to a gas, and it occurs at the boiling point of the substance. In the case of water, the boiling point is 100 degrees Celsius (212 degrees Fahrenheit) at atmospheric pressure.
During the process of vaporization, the heat energy supplied to the water is used to break the intermolecular forces between the water molecules, allowing them to escape into the surrounding air as water vapor. This process requires a large amount of energy, known as the latent heat of vaporization.
As the heat energy is absorbed by the water molecules and used for vaporization, the temperature of the water remains constant at the boiling point. This is because the energy is being used to change the state of the water rather than increase its temperature.
Once all the liquid water has been converted into water vapor, the temperature of the water can start to rise again as the heat energy supplied by the burner is no longer being used for vaporization.
Summary:
When heat is constantly supplied by a burner to boiling water, the temperature of the water does not rise at all. This is because the heat energy is used for the process of vaporization, converting the liquid water into water vapor, rather than increasing the temperature of the water.
The evaporation of water increases under the following conditions- a)Increase in surface area, decrease in temperature
- b)Increase in surface area, rise in temperature
- c)Increase in temperature, decrease in surface area
- d)Increase in temperature, increase in surface area addition of common salt
Correct answer is option 'B'. Can you explain this answer?
The evaporation of water increases under the following conditions
a)
Increase in surface area, decrease in temperature
b)
Increase in surface area, rise in temperature
c)
Increase in temperature, decrease in surface area
d)
Increase in temperature, increase in surface area addition of common salt
|
|
Shilpa Choudhury answered |
When surface area increases and the temperature rises, evaporation of water also increases, because the area which is exposed to the outer atmosphere is more and increasing the temperature, leads to an increase in the kinetic energy, due to which the rate of evaporation also increases.
What will be boiling point of water at hill station?- a)100°C
- b)<100°C
- c)>100°C
- d)Either less than 100°C
Correct answer is option 'B'. Can you explain this answer?
What will be boiling point of water at hill station?
a)
100°C
b)
<100°C
c)
>100°C
d)
Either less than 100°C
|
|
Shilpa Choudhury answered |
The boiling point is the temperature at which the vapour pressure of the liquid equals the environmental pressure surrounding the liquid. Atmospheric pressure is due to air above any given point. The atmospheric pressure at high altitudes like hill station is less than at the sea level.Thus, vapour pressure will equal atmospheric pressure at a comparatively low temperature. Thus, the boiling point of water is reduced less than at sea level or <100°C.
Which of the following has minimum kinetic energy?
- a)Particles of steam at 100
- b)Particles of water at 0
- c)Particles of water at 100
- d)Particles of ice below 0
Correct answer is option 'D'. Can you explain this answer?
Which of the following has minimum kinetic energy?
a)
Particles of steam at 100
b)
Particles of water at 0
c)
Particles of water at 100
d)
Particles of ice below 0
|
|
Sameer Menon answered |
Understanding Kinetic Energy in States of Matter
Kinetic energy is the energy of motion, and in the context of particles in different states of matter, it varies significantly depending on temperature and state.
States of Matter and Temperature
- **Steam at 100°C:**
- Particles are in a gaseous state, moving rapidly and freely.
- High kinetic energy due to increased temperature.
- **Water at 0°C:**
- Particles are in a liquid state, moving but less freely than in gas.
- Moderate kinetic energy.
- **Water at 100°C:**
- Particles are in a liquid state, but transitioning to gas.
- Higher kinetic energy, similar to steam.
- **Ice below 0°C:**
- Particles are in a solid state, vibrating in fixed positions.
- Minimal kinetic energy as the temperature is low.
Why Ice Below 0°C Has Minimum Kinetic Energy
- **Solid State Characteristics:**
- In solids like ice, particles are closely packed and primarily vibrate in place rather than moving freely.
- **Low Temperature Effect:**
- Below 0°C, the temperature decreases the energy of the particles, resulting in lower kinetic energy.
- **Comparison with Other States:**
- The kinetic energy of particles in steam and water (both at higher temperatures) is significantly greater than that of ice, making ice the state with minimum kinetic energy.
Conclusion
In summary, the particles of ice below 0°C have the minimum kinetic energy among the given options due to their solid-state arrangement and lower temperature, which restricts their motion compared to liquids and gases at higher temperatures.
Kinetic energy is the energy of motion, and in the context of particles in different states of matter, it varies significantly depending on temperature and state.
States of Matter and Temperature
- **Steam at 100°C:**
- Particles are in a gaseous state, moving rapidly and freely.
- High kinetic energy due to increased temperature.
- **Water at 0°C:**
- Particles are in a liquid state, moving but less freely than in gas.
- Moderate kinetic energy.
- **Water at 100°C:**
- Particles are in a liquid state, but transitioning to gas.
- Higher kinetic energy, similar to steam.
- **Ice below 0°C:**
- Particles are in a solid state, vibrating in fixed positions.
- Minimal kinetic energy as the temperature is low.
Why Ice Below 0°C Has Minimum Kinetic Energy
- **Solid State Characteristics:**
- In solids like ice, particles are closely packed and primarily vibrate in place rather than moving freely.
- **Low Temperature Effect:**
- Below 0°C, the temperature decreases the energy of the particles, resulting in lower kinetic energy.
- **Comparison with Other States:**
- The kinetic energy of particles in steam and water (both at higher temperatures) is significantly greater than that of ice, making ice the state with minimum kinetic energy.
Conclusion
In summary, the particles of ice below 0°C have the minimum kinetic energy among the given options due to their solid-state arrangement and lower temperature, which restricts their motion compared to liquids and gases at higher temperatures.
When a gas jar full of air is placed upside down on a gas jar full of bromine vapours, the red–brown vapours of bromine from the lower jar go upward into the jar containing air. In this experiment- a)Bromine is heavier than air
- b)Air is heavier than bromine
- c)Coth air and bromine have the same density
- d)Bromine cannot be heavier than air because it is going upwards against gravity
Correct answer is option 'A'. Can you explain this answer?
When a gas jar full of air is placed upside down on a gas jar full of bromine vapours, the red–brown vapours of bromine from the lower jar go upward into the jar containing air. In this experiment
a)
Bromine is heavier than air
b)
Air is heavier than bromine
c)
Coth air and bromine have the same density
d)
Bromine cannot be heavier than air because it is going upwards against gravity
|
|
Priya Choudhary answered |
Explanation:
When a gas jar full of air is placed upside down on a gas jar full of bromine vapours, the red–brown vapours of bromine from the lower jar go upward into the jar containing air. This phenomenon can be explained by the difference in density between bromine and air.
Density of Bromine:
Bromine is a dense liquid, with a density of about 3.1 grams per cubic centimeter. This means that a given volume of bromine is much heavier than the same volume of air.
Density of Air:
Air, on the other hand, is a mixture of gases, primarily nitrogen (N2) and oxygen (O2), with a density of about 1.2 grams per cubic centimeter. This means that a given volume of air is lighter than the same volume of bromine.
Why does bromine go upward into the jar containing air?
The movement of bromine vapours from the lower jar into the jar containing air can be explained by the principle of diffusion. Diffusion is the process by which particles move from an area of higher concentration to an area of lower concentration.
In this case, the jar containing bromine vapours has a higher concentration of bromine molecules compared to the jar containing air. As a result, the bromine molecules tend to spread out or diffuse into the jar with lower bromine concentration, which is the jar containing air.
Since bromine is heavier than air, it might be expected that the bromine vapours would sink down instead of rising up. However, diffusion is driven by the random motion of particles, and the motion of gas particles is influenced by factors such as temperature and pressure.
In this experiment, the movement of bromine vapours upwards against gravity can be explained by the fact that the bromine molecules are highly volatile and have a higher vapor pressure compared to the air molecules. As a result, the bromine molecules are able to overcome the force of gravity and rise up into the jar containing air.
Therefore, the correct answer to the question is option 'A': Bromine is heavier than air.
When a gas jar full of air is placed upside down on a gas jar full of bromine vapours, the red–brown vapours of bromine from the lower jar go upward into the jar containing air. This phenomenon can be explained by the difference in density between bromine and air.
Density of Bromine:
Bromine is a dense liquid, with a density of about 3.1 grams per cubic centimeter. This means that a given volume of bromine is much heavier than the same volume of air.
Density of Air:
Air, on the other hand, is a mixture of gases, primarily nitrogen (N2) and oxygen (O2), with a density of about 1.2 grams per cubic centimeter. This means that a given volume of air is lighter than the same volume of bromine.
Why does bromine go upward into the jar containing air?
The movement of bromine vapours from the lower jar into the jar containing air can be explained by the principle of diffusion. Diffusion is the process by which particles move from an area of higher concentration to an area of lower concentration.
In this case, the jar containing bromine vapours has a higher concentration of bromine molecules compared to the jar containing air. As a result, the bromine molecules tend to spread out or diffuse into the jar with lower bromine concentration, which is the jar containing air.
Since bromine is heavier than air, it might be expected that the bromine vapours would sink down instead of rising up. However, diffusion is driven by the random motion of particles, and the motion of gas particles is influenced by factors such as temperature and pressure.
In this experiment, the movement of bromine vapours upwards against gravity can be explained by the fact that the bromine molecules are highly volatile and have a higher vapor pressure compared to the air molecules. As a result, the bromine molecules are able to overcome the force of gravity and rise up into the jar containing air.
Therefore, the correct answer to the question is option 'A': Bromine is heavier than air.
Ice floats on water because- a)It is solid
- b)It is low melting solid
- c)It has higher density than water
- d)It has lower density than water due to more volume
Correct answer is option 'D'. Can you explain this answer?
Ice floats on water because
a)
It is solid
b)
It is low melting solid
c)
It has higher density than water
d)
It has lower density than water due to more volume
|
|
Shilpa Choudhury answered |
When water freezes into its solid form, the molecules can form more stable hydrogen bonds, locking them into positions. As the molecules are not moving, they cannot form as many hydrogen bonds as other water molecules. This leads to ice water molecules not being as close together as in liquid water, thus reducing its density.
Seema took a 100 mL beaker and filled half the beaker with water and marked the level of water.She dissolved some salt with the help of a glass rod and recorded water level again. Choose the correct observation related to above activity- a)The water level decreases
- b)There is little increase in water level
- c)The water level remains the same
- d)The water level increases appreciably
Correct answer is option 'C'. Can you explain this answer?
Seema took a 100 mL beaker and filled half the beaker with water and marked the level of water.
She dissolved some salt with the help of a glass rod and recorded water level again. Choose the correct observation related to above activity
a)
The water level decreases
b)
There is little increase in water level
c)
The water level remains the same
d)
The water level increases appreciably
|
|
Shilpa Choudhury answered |
When salt is dissolved in water, the particles of salt disappear in water. This happen because particles of salt get adjusted in the space b/w the particles of water.
Additionally, you will notice that there is no rise of water level take place when one or more teaspoons of salt is added in the glass of water, this is because salt particles get adjusted in the space b/w the particles of water and no rise in the water level comes in result.
Helium gas is a matter because- a)It has mass and occupies volume
- b)It has no definite volume
- c)It can be compressed easily
- d)It has a mass
Correct answer is option 'A'. Can you explain this answer?
Helium gas is a matter because
a)
It has mass and occupies volume
b)
It has no definite volume
c)
It can be compressed easily
d)
It has a mass
|
|
Sameer Chavan answered |
Understanding Helium Gas as Matter
Helium gas is classified as matter due to its fundamental properties. Here's a detailed breakdown:
Mass and Volume
- Helium has a definite mass, even though it is a light gas.
- All matter possesses mass, which is a key characteristic that defines it as matter.
- Additionally, helium occupies space, which is another essential property of matter.
Definite Volume
- Although helium gas does not have a definite volume in the sense of a fixed shape, it still occupies space within a container.
- Gases expand to fill the volume of their containers, but they are still considered to have volume since they displace air and other gases.
Compressibility
- Helium can be compressed easily, which is a characteristic of gases.
- While compressibility is notable, it does not define matter; rather, it describes the behavior of gases.
Conclusion
- The key reason helium is classified as matter is its mass and the ability to occupy space.
- Thus, the correct answer is option 'A': "It has mass and occupies volume," highlighting the essential properties of all matter.
Helium gas is classified as matter due to its fundamental properties. Here's a detailed breakdown:
Mass and Volume
- Helium has a definite mass, even though it is a light gas.
- All matter possesses mass, which is a key characteristic that defines it as matter.
- Additionally, helium occupies space, which is another essential property of matter.
Definite Volume
- Although helium gas does not have a definite volume in the sense of a fixed shape, it still occupies space within a container.
- Gases expand to fill the volume of their containers, but they are still considered to have volume since they displace air and other gases.
Compressibility
- Helium can be compressed easily, which is a characteristic of gases.
- While compressibility is notable, it does not define matter; rather, it describes the behavior of gases.
Conclusion
- The key reason helium is classified as matter is its mass and the ability to occupy space.
- Thus, the correct answer is option 'A': "It has mass and occupies volume," highlighting the essential properties of all matter.
During respiration, glucose and oxygen enter our body cells whereas waste products like carbondioxide and water leave the body cells by the process of- a)Osmosis
- b)Effusion
- c)Diffusion
- d)Plasmolysis
Correct answer is option 'C'. Can you explain this answer?
During respiration, glucose and oxygen enter our body cells whereas waste products like carbon
dioxide and water leave the body cells by the process of
a)
Osmosis
b)
Effusion
c)
Diffusion
d)
Plasmolysis
|
|
Shilpa Choudhury answered |
Breathing involves the exchange of gases in the lungs, a process which occurs by diffusion. The oxygen is then transported throughout the body. Carbon dioxide is the waste gas produced by respiration.
The mixture of sulphur and sodium chloride can be separated by- a)Dissolving in water followed by filtration and evaporation
- b)Sublimation
- c)Dissolving in alcohol followed by filtration
- d)Crystallization.
Correct answer is option 'A'. Can you explain this answer?
The mixture of sulphur and sodium chloride can be separated by
a)
Dissolving in water followed by filtration and evaporation
b)
Sublimation
c)
Dissolving in alcohol followed by filtration
d)
Crystallization.
|
|
Zara Khan answered |
Separation of sulphur and sodium chloride mixture
Introduction:
The mixture of sulphur and sodium chloride can be separated by various methods based on the physical properties of the components. One of the most effective methods is to dissolve the mixture in water followed by filtration and evaporation.
Method:
The following steps can be followed to separate sulphur and sodium chloride using this method:
1. Dissolving in water:
- The mixture of sulphur and sodium chloride is added to a beaker containing water.
- Both sulphur and sodium chloride are soluble in water, so they dissolve in the solvent.
2. Filtration:
- Once the mixture is dissolved, it is poured through a filter paper placed in a funnel.
- The filter paper allows the water to pass through, but retains the solid particles.
3. Evaporation:
- The filtrate containing the dissolved sodium chloride and sulphur is collected in a separate container.
- The water is then evaporated from the filtrate using a heating source like a Bunsen burner or hot plate.
- As the water evaporates, the dissolved sodium chloride and sulphur crystallize and separate from the solution.
4. Separation:
- The solid crystals of sodium chloride and sulphur can be separated from each other using various methods such as decantation or filtration.
- Decantation can be used if the crystals are large and settle at the bottom of the container. The liquid can be carefully poured out, leaving the solid behind.
- Filtration can be used if the crystals are small and mixed with impurities. The crystals can be collected on a filter paper and washed with a suitable solvent to remove impurities.
Conclusion:
By dissolving the mixture of sulphur and sodium chloride in water, followed by filtration and evaporation, we can separate the two components. This method takes advantage of the difference in solubility of sulphur and sodium chloride in water. While sodium chloride is highly soluble, sulphur has limited solubility and can be easily separated from the solution.
Introduction:
The mixture of sulphur and sodium chloride can be separated by various methods based on the physical properties of the components. One of the most effective methods is to dissolve the mixture in water followed by filtration and evaporation.
Method:
The following steps can be followed to separate sulphur and sodium chloride using this method:
1. Dissolving in water:
- The mixture of sulphur and sodium chloride is added to a beaker containing water.
- Both sulphur and sodium chloride are soluble in water, so they dissolve in the solvent.
2. Filtration:
- Once the mixture is dissolved, it is poured through a filter paper placed in a funnel.
- The filter paper allows the water to pass through, but retains the solid particles.
3. Evaporation:
- The filtrate containing the dissolved sodium chloride and sulphur is collected in a separate container.
- The water is then evaporated from the filtrate using a heating source like a Bunsen burner or hot plate.
- As the water evaporates, the dissolved sodium chloride and sulphur crystallize and separate from the solution.
4. Separation:
- The solid crystals of sodium chloride and sulphur can be separated from each other using various methods such as decantation or filtration.
- Decantation can be used if the crystals are large and settle at the bottom of the container. The liquid can be carefully poured out, leaving the solid behind.
- Filtration can be used if the crystals are small and mixed with impurities. The crystals can be collected on a filter paper and washed with a suitable solvent to remove impurities.
Conclusion:
By dissolving the mixture of sulphur and sodium chloride in water, followed by filtration and evaporation, we can separate the two components. This method takes advantage of the difference in solubility of sulphur and sodium chloride in water. While sodium chloride is highly soluble, sulphur has limited solubility and can be easily separated from the solution.
When we determine the boiling point of liquid the thermometer- a)Should dip into liquid
- b)Should be above the liquid and remain vertical
- c)Should touch the bottom of container
- d)Should be placed slanting in the liquid
Correct answer is option 'B'. Can you explain this answer?
When we determine the boiling point of liquid the thermometer
a)
Should dip into liquid
b)
Should be above the liquid and remain vertical
c)
Should touch the bottom of container
d)
Should be placed slanting in the liquid
|
|
Zara Khan answered |
Answer:
Introduction:
When determining the boiling point of a liquid using a thermometer, it is important to follow certain guidelines to ensure accurate measurements. The correct option in this case is option 'B', which states that the thermometer should be above the liquid and remain vertical. Let's explore why this is the correct choice.
Explanation:
To measure the boiling point of a liquid, the thermometer should be positioned in a way that allows it to accurately measure the temperature of the vapor being produced. Placing the thermometer in different positions can lead to inaccurate readings. Here's why option 'B' is the correct choice:
Above the liquid:
Placing the thermometer above the liquid ensures that it measures the temperature of the vapor rather than being influenced by the temperature of the liquid itself. The boiling point of a liquid is the temperature at which its vapor pressure equals the atmospheric pressure. By positioning the thermometer above the liquid, it can accurately measure the temperature of the vapor as it escapes from the liquid.
Remain vertical:
Keeping the thermometer vertical ensures that it is immersed in the rising vapor and not tilted towards any particular direction. This helps in obtaining a more precise reading as the thermometer is evenly exposed to the vapor and can measure its temperature uniformly. Tilting the thermometer could lead to uneven temperature measurements, affecting the accuracy of the boiling point determination.
Other options:
- Option 'A': Dipping the thermometer into the liquid would measure the temperature of the liquid itself, not the boiling point. This would not provide accurate results.
- Option 'C': Touching the bottom of the container could lead to the thermometer measuring the temperature of the container rather than the vapor. This would result in inaccurate readings.
- Option 'D': Placing the thermometer slanting in the liquid would likely lead to inaccurate measurements as the thermometer would not be fully immersed in the rising vapor.
Conclusion:
To determine the boiling point of a liquid accurately, it is important to position the thermometer above the liquid and keep it vertical. This ensures that the thermometer measures the temperature of the vapor being produced, leading to more precise and reliable results.
Introduction:
When determining the boiling point of a liquid using a thermometer, it is important to follow certain guidelines to ensure accurate measurements. The correct option in this case is option 'B', which states that the thermometer should be above the liquid and remain vertical. Let's explore why this is the correct choice.
Explanation:
To measure the boiling point of a liquid, the thermometer should be positioned in a way that allows it to accurately measure the temperature of the vapor being produced. Placing the thermometer in different positions can lead to inaccurate readings. Here's why option 'B' is the correct choice:
Above the liquid:
Placing the thermometer above the liquid ensures that it measures the temperature of the vapor rather than being influenced by the temperature of the liquid itself. The boiling point of a liquid is the temperature at which its vapor pressure equals the atmospheric pressure. By positioning the thermometer above the liquid, it can accurately measure the temperature of the vapor as it escapes from the liquid.
Remain vertical:
Keeping the thermometer vertical ensures that it is immersed in the rising vapor and not tilted towards any particular direction. This helps in obtaining a more precise reading as the thermometer is evenly exposed to the vapor and can measure its temperature uniformly. Tilting the thermometer could lead to uneven temperature measurements, affecting the accuracy of the boiling point determination.
Other options:
- Option 'A': Dipping the thermometer into the liquid would measure the temperature of the liquid itself, not the boiling point. This would not provide accurate results.
- Option 'C': Touching the bottom of the container could lead to the thermometer measuring the temperature of the container rather than the vapor. This would result in inaccurate readings.
- Option 'D': Placing the thermometer slanting in the liquid would likely lead to inaccurate measurements as the thermometer would not be fully immersed in the rising vapor.
Conclusion:
To determine the boiling point of a liquid accurately, it is important to position the thermometer above the liquid and keep it vertical. This ensures that the thermometer measures the temperature of the vapor being produced, leading to more precise and reliable results.
Which of the following statement is incorrect?- a)The particles of matter are very-very small
- b)The particles of matter attract one another
- c)The particles of all the matter have spaces between them
- d)The particles of some of the matter are moving constantly
Correct answer is option 'D'. Can you explain this answer?
Which of the following statement is incorrect?
a)
The particles of matter are very-very small
b)
The particles of matter attract one another
c)
The particles of all the matter have spaces between them
d)
The particles of some of the matter are moving constantly
|
|
Shilpa Choudhury answered |
The important characteristics of particles of matter are the following
(i) The particles of matter are very small.
(ii) The particles of matter attract each other.
(iii) The particles of matter have space between them.
(iv) All the particles of matter are constantly moving.
Also, particles are not stable, they all have some kinetic energy so move constantly.
So, option C is incorrect.
Which of the following is correct arrangement of decreasing order of force of attraction betweenparticles?- a)Water, air, chalk
- b)Sugar, alcohol, nitrogen
- c)Sulphur dioxide, carbon disulphide, sulphur
- d)Oxygen, water sugar.
Correct answer is option 'B'. Can you explain this answer?
Which of the following is correct arrangement of decreasing order of force of attraction between
particles?
a)
Water, air, chalk
b)
Sugar, alcohol, nitrogen
c)
Sulphur dioxide, carbon disulphide, sulphur
d)
Oxygen, water sugar.
|
|
Avani Shah answered |
Decreasing Order of Force of Attraction Between Particles
Sugar, alcohol, and nitrogen have intermolecular forces of attraction that are stronger than those between water, air, and chalk. Sulphur dioxide, carbon disulphide, and sulphur have even stronger forces of attraction than the former three, while oxygen, water, and sugar have the weakest forces of attraction.
Explanation:
Intermolecular forces of attraction refer to the forces that hold the particles of a substance together. These forces include Van der Waals forces, dipole-dipole interactions, and hydrogen bonding. The strength of these forces varies depending on the type of particles involved and their arrangement.
The correct arrangement of decreasing order of force of attraction between particles is option B (sugar, alcohol, nitrogen). Sugar and alcohol have strong intermolecular forces of attraction due to the presence of hydrogen bonding between their molecules. Nitrogen also has strong intermolecular forces of attraction due to the presence of Van der Waals forces between its molecules.
Option A (water, air, chalk) has weaker intermolecular forces of attraction than option B. Water has hydrogen bonding between its molecules, but air and chalk have weaker Van der Waals forces between their particles.
Option C (sulphur dioxide, carbon disulphide, sulphur) has even stronger intermolecular forces of attraction than option B. Sulphur dioxide and carbon disulphide have dipole-dipole interactions and Van der Waals forces between their molecules, while sulphur has strong Van der Waals forces between its particles.
Option D (oxygen, water, sugar) has the weakest intermolecular forces of attraction among the given options. Oxygen has weak Van der Waals forces between its molecules, while water and sugar have weaker hydrogen bonding than sugar and alcohol.
In summary, the correct arrangement of decreasing order of force of attraction between particles is:
1. Sulphur dioxide, carbon disulphide, sulphur
2. Sugar, alcohol, nitrogen
3. Water, air, chalk
4. Oxygen, water, sugar.
Sugar, alcohol, and nitrogen have intermolecular forces of attraction that are stronger than those between water, air, and chalk. Sulphur dioxide, carbon disulphide, and sulphur have even stronger forces of attraction than the former three, while oxygen, water, and sugar have the weakest forces of attraction.
Explanation:
Intermolecular forces of attraction refer to the forces that hold the particles of a substance together. These forces include Van der Waals forces, dipole-dipole interactions, and hydrogen bonding. The strength of these forces varies depending on the type of particles involved and their arrangement.
The correct arrangement of decreasing order of force of attraction between particles is option B (sugar, alcohol, nitrogen). Sugar and alcohol have strong intermolecular forces of attraction due to the presence of hydrogen bonding between their molecules. Nitrogen also has strong intermolecular forces of attraction due to the presence of Van der Waals forces between its molecules.
Option A (water, air, chalk) has weaker intermolecular forces of attraction than option B. Water has hydrogen bonding between its molecules, but air and chalk have weaker Van der Waals forces between their particles.
Option C (sulphur dioxide, carbon disulphide, sulphur) has even stronger intermolecular forces of attraction than option B. Sulphur dioxide and carbon disulphide have dipole-dipole interactions and Van der Waals forces between their molecules, while sulphur has strong Van der Waals forces between its particles.
Option D (oxygen, water, sugar) has the weakest intermolecular forces of attraction among the given options. Oxygen has weak Van der Waals forces between its molecules, while water and sugar have weaker hydrogen bonding than sugar and alcohol.
In summary, the correct arrangement of decreasing order of force of attraction between particles is:
1. Sulphur dioxide, carbon disulphide, sulphur
2. Sugar, alcohol, nitrogen
3. Water, air, chalk
4. Oxygen, water, sugar.
When water at 0°C freezes to form ice at the same temperature of 0°C, then it- a)Absorbs some heat
- b)Release some heat
- c)Neither absorbs nor releases heat
- d)Absorbs exactly 3.34 × 105 j/kg of heat
Correct answer is option 'B'. Can you explain this answer?
When water at 0°C freezes to form ice at the same temperature of 0°C, then it
a)
Absorbs some heat
b)
Release some heat
c)
Neither absorbs nor releases heat
d)
Absorbs exactly 3.34 × 105 j/kg of heat
|
|
Shilpa Choudhury answered |
When the liquid water turns to solid ice we find that water freezes without getting any colder. That's because the latent heat of fusion is being lost from the liquid as it solidifies and the temperature of the water does not fall so quickly.
Which one of the following statement is correct in respect of fluids?- a)Only gases behave as fluids
- b)Only liquids are fluids
- c)Gases and solids behave as fluids
- d)Gases and liquids behave as fluids
Correct answer is option 'D'. Can you explain this answer?
Which one of the following statement is correct in respect of fluids?
a)
Only gases behave as fluids
b)
Only liquids are fluids
c)
Gases and solids behave as fluids
d)
Gases and liquids behave as fluids
|
|
Shilpa Choudhury answered |
Fluids are the substances that have the ability to flow. Gases and liquids can flow easily because their particles are free to move. Hence, gases and liquids behave as fluids is the correct option.
In the following diagram ammonia gas obtained from ammonium hydroxide reacts with HCl (g) obtained from hydrochloric acid, and form white fumes of ammonium chloride. Observe the following diagram and answer the following question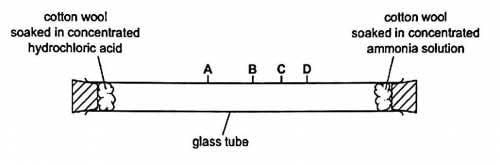
- a)White fumes ring will be formed at the centre
- b)White fumes ring will be formed near 2 end
- c)White fumes ring will be formed near 1 end
- d)White fumes ring will not be formed at all
Correct answer is option 'B'. Can you explain this answer?
In the following diagram ammonia gas obtained from ammonium hydroxide reacts with HCl (g) obtained from hydrochloric acid, and form white fumes of ammonium chloride. Observe the following diagram and answer the following question

a)
White fumes ring will be formed at the centre
b)
White fumes ring will be formed near 2 end
c)
White fumes ring will be formed near 1 end
d)
White fumes ring will not be formed at all
|
|
Shilpa Choudhury answered |
Ammonia is a weak base and it combines with HCl to form ammonium chloride. But moisture is necessary to bring out this reaction. So when exposed to air they combine to form dense fumes of ammonium chloride. The salts produced by the action of ammonia on acids are known as the ammonium salts and all contain the ammonium ion.
Which of the following factors are responsible for the change in state of solid carbon dioxide when kept exposed to air?(i) Increase in pressure (ii) Increase in temperature(iii) Decrease in pressure (iv) Decrease in temperature- a)(i) and (ii)
- b)(ii) and (iii)
- c)(i) and (iii)
- d)(ii) and (iv)
Correct answer is option 'B'. Can you explain this answer?
Which of the following factors are responsible for the change in state of solid carbon dioxide when kept exposed to air?
(i) Increase in pressure (ii) Increase in temperature
(iii) Decrease in pressure (iv) Decrease in temperature
a)
(i) and (ii)
b)
(ii) and (iii)
c)
(i) and (iii)
d)
(ii) and (iv)
|
|
Shilpa Choudhury answered |
Solid carbon dioxide can be changed into vapour or gaseous state by decreasing the pressure and increasing the temperature. Both the processes increase the inter-particle spaces in the solid carbon dioxide.
For any substance the temperature remains same during the change of state due to- a)Loss of heat
- b)Latent heat
- c)Less supply of heat
- d)Lattice energy
Correct answer is option 'B'. Can you explain this answer?
For any substance the temperature remains same during the change of state due to
a)
Loss of heat
b)
Latent heat
c)
Less supply of heat
d)
Lattice energy
|
|
Shilpa Choudhury answered |
Temperature remains constant during change of state
It is due to the latent heat as the heat supplied to increase the temperature of the substance is used up to transform the state of matter of the substance hence the temperature stays constant.
Hence the temperature remains constant as all the heat is used up and no external heat is released or absorbed.
One of the following does not undergo sublimation. This one is- a)Iodine
- b)Camphor
- c)Sodium chloride
- d)Ammonium chloride
Correct answer is option 'C'. Can you explain this answer?
One of the following does not undergo sublimation. This one is
a)
Iodine
b)
Camphor
c)
Sodium chloride
d)
Ammonium chloride
|
|
Shilpa Choudhury answered |
Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Iodine and ammonium chloride produces fumes on gentle heating while sodium chloride does not sublime.
During summer days water kept in an earthen pot (pitcher) becomes cool because of the phenomenonof- a)Osmosis
- b)Evaporation
- c)Transpiration
- d)Diffusion
Correct answer is option 'B'. Can you explain this answer?
During summer days water kept in an earthen pot (pitcher) becomes cool because of the phenomenon
of
a)
Osmosis
b)
Evaporation
c)
Transpiration
d)
Diffusion
|
|
Shilpa Choudhury answered |
An earthen pot (matka) has small pores in it. Water seeps through these pores and reaches the outer surface of the earthen pot. This water then evaporates by taking heat from the earthen pot, thereby making it cooler.
Which of the following energy is absorbed during the change of state of a substance?- a)Heat capacity
- b)Latent heat
- c)Heat of solution
- d)Specific heat
Correct answer is option 'B'. Can you explain this answer?
Which of the following energy is absorbed during the change of state of a substance?
a)
Heat capacity
b)
Latent heat
c)
Heat of solution
d)
Specific heat
|
|
Shilpa Choudhury answered |
Latent heat is absorbed during the change of state of a substance because it is the heat energy that has to be supplied to change the state of a substance, while the specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
The heat capacity of a system is defined as the amount of heat needed to raise the system's temperature by one degree.
Convert the temperature of 300 k to the Celsius scale- a)30°C
- b)27°C
- c)28°C
- d)26°C
Correct answer is option ''. Can you explain this answer?
Convert the temperature of 300 k to the Celsius scale
a)
30°C
b)
27°C
c)
28°C
d)
26°C
|
|
Shilpa Choudhury answered |
Temperature on Celsius scale = Temperature
on Kelvin scale – 273
= 300 – 273
= 27°C
Particles of matter are- a)Stationary
- b)In continuous motion
- c)Rotating about on-axis
- d)Vibrating in one position
Correct answer is option 'B'. Can you explain this answer?
Particles of matter are
a)
Stationary
b)
In continuous motion
c)
Rotating about on-axis
d)
Vibrating in one position
|
|
Shilpa Choudhury answered |
Particles of matter are continuously moving.
For example, the smell of deodorant travels in the whole room if it is used on one side of the room.
Hence, the correct option is B.
If the temperature of an object is 268 k, it will be equivalent to- a)–5°C
- b)+5°C
- c)–25°C
- d)368°C
Correct answer is option 'A'. Can you explain this answer?
If the temperature of an object is 268 k, it will be equivalent to
a)
–5°C
b)
+5°C
c)
–25°C
d)
368°C
|
|
Shilpa Choudhury answered |
Temperature on Celsius scale = Temperature
on Kelvin scale + 273
= 268 – 273
= – 5°C
Chapter doubts & questions for Matter in Our Surroundings - Science Olympiad Class 9 2025 is part of Class 9 exam preparation. The chapters have been prepared according to the Class 9 exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Class 9 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Matter in Our Surroundings - Science Olympiad Class 9 in English & Hindi are available as part of Class 9 exam.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Science Olympiad Class 9
28 videos|115 docs|52 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









