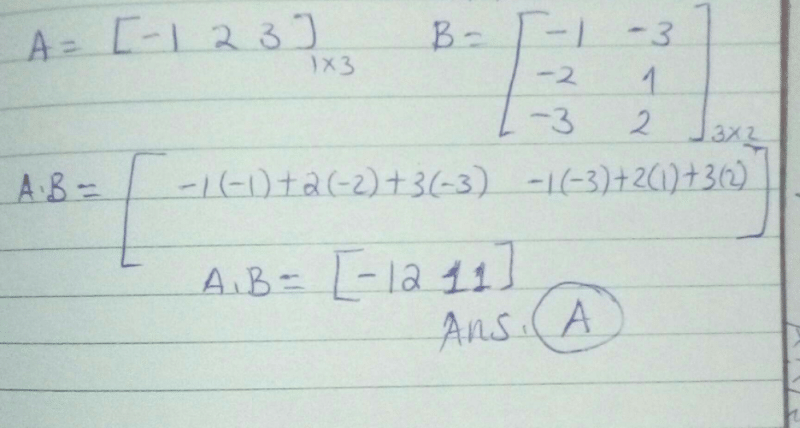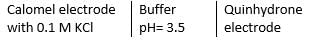All Exams >
JEE >
Weekly Tests for JEE Preparation >
All Questions
All questions of May Week 4 for JEE Exam
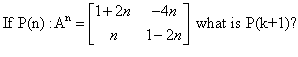
- a)
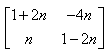
- b)
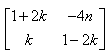
- c)
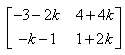
- d)
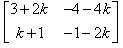
Correct answer is option 'D'. Can you explain this answer?
a)
b)
c)
d)

|
Sushil Kumar answered |
P(n) : An = {(1+2n, -4n), (n,(1 - 2n))}
= P(k + 1) = {(1+2(k+1), -4(k+1)), (k+1, (1 - 2(k+1)}
= {(1+2k+2, -4k-4) (k+1, 1-2k-2)}
= {(2k+3, -4k-4), (k+1, -2k-1)}
= P(k + 1) = {(1+2(k+1), -4(k+1)), (k+1, (1 - 2(k+1)}
= {(1+2k+2, -4k-4) (k+1, 1-2k-2)}
= {(2k+3, -4k-4), (k+1, -2k-1)}
If 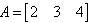 and
and 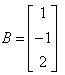 , then AB=?
, then AB=?- a)[7]
- b)[1 - 12]
- c)

- d)[18]
Correct answer is option 'A'. Can you explain this answer?
If 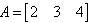 and
and 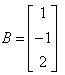 , then AB=?
, then AB=?
a)
[7]
b)
[1 - 12]
c)
d)
[18]
|
|
Ritu Singh answered |
A = [2, 3, 4]
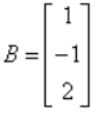 Therefore AXB = {(2*1) + (3*(-1)) + (4*2)}
Therefore AXB = {(2*1) + (3*(-1)) + (4*2)}
AXB = {2 + (-3) + 8}
AXB = 7
 Therefore AXB = {(2*1) + (3*(-1)) + (4*2)}
Therefore AXB = {(2*1) + (3*(-1)) + (4*2)}AXB = {2 + (-3) + 8}
AXB = 7
For a skew symmetric even ordered matrix A of integers, which of the following will not hold true:
- a)det(A) = 9
- b)det(A) = 81
- c)det(A) = 7
- d)det(A) = 4
Correct answer is option 'C'. Can you explain this answer?
For a skew symmetric even ordered matrix A of integers, which of the following will not hold true:
a)
det(A) = 9
b)
det(A) = 81
c)
det(A) = 7
d)
det(A) = 4
|
|
Aniket Dasgupta answered |
Skew Symmetric Even Ordered Matrix and Determinant
Skew Symmetric Matrix:
A skew symmetric matrix is a square matrix whose transpose is equal to its negative. In other words, if A is a skew symmetric matrix, then A^T = -A.
Example:
[0 -3 4]
[3 0 -5]
[-4 5 0]
This is a 3x3 skew symmetric matrix because A^T = -A.
Even Ordered Matrix:
An even ordered matrix is a square matrix whose order is even. In other words, if A is an even ordered matrix, then the order of A is 2n, where n is a positive integer.
Example:
[2 1 5 3]
[4 6 8 2]
[9 7 1 5]
[3 4 2 6]
This is a 4x4 even ordered matrix because the order of A is 2n=4.
Determinant of a Skew Symmetric Even Ordered Matrix:
The determinant of a skew symmetric even ordered matrix is always equal to zero. This is because the determinant of a skew symmetric matrix of odd order is always equal to zero and the determinant of any even ordered matrix can be expressed as a sum of permutations of the determinants of its n x n submatrices. Since the submatrices of a skew symmetric matrix are also skew symmetric, their determinants are equal to zero. Therefore, the determinant of a skew symmetric even ordered matrix is also equal to zero.
Solution:
a) det(A) = 9
Since the determinant of a skew symmetric even ordered matrix is always equal to zero, this statement is false. Therefore, option 'a' is not true.
b) det(A) = 81
Since the determinant of a skew symmetric even ordered matrix is always equal to zero, this statement is false. Therefore, option 'b' is not true.
c) det(A) = 7
This statement is false because the determinant of a skew symmetric even ordered matrix is always equal to zero.
d) det(A) = 4
Since the determinant of a skew symmetric even ordered matrix is always equal to zero, this statement is false. Therefore, option 'd' is not true.
Therefore, the correct answer is option 'c'.
Skew Symmetric Matrix:
A skew symmetric matrix is a square matrix whose transpose is equal to its negative. In other words, if A is a skew symmetric matrix, then A^T = -A.
Example:
[0 -3 4]
[3 0 -5]
[-4 5 0]
This is a 3x3 skew symmetric matrix because A^T = -A.
Even Ordered Matrix:
An even ordered matrix is a square matrix whose order is even. In other words, if A is an even ordered matrix, then the order of A is 2n, where n is a positive integer.
Example:
[2 1 5 3]
[4 6 8 2]
[9 7 1 5]
[3 4 2 6]
This is a 4x4 even ordered matrix because the order of A is 2n=4.
Determinant of a Skew Symmetric Even Ordered Matrix:
The determinant of a skew symmetric even ordered matrix is always equal to zero. This is because the determinant of a skew symmetric matrix of odd order is always equal to zero and the determinant of any even ordered matrix can be expressed as a sum of permutations of the determinants of its n x n submatrices. Since the submatrices of a skew symmetric matrix are also skew symmetric, their determinants are equal to zero. Therefore, the determinant of a skew symmetric even ordered matrix is also equal to zero.
Solution:
a) det(A) = 9
Since the determinant of a skew symmetric even ordered matrix is always equal to zero, this statement is false. Therefore, option 'a' is not true.
b) det(A) = 81
Since the determinant of a skew symmetric even ordered matrix is always equal to zero, this statement is false. Therefore, option 'b' is not true.
c) det(A) = 7
This statement is false because the determinant of a skew symmetric even ordered matrix is always equal to zero.
d) det(A) = 4
Since the determinant of a skew symmetric even ordered matrix is always equal to zero, this statement is false. Therefore, option 'd' is not true.
Therefore, the correct answer is option 'c'.
The product of two matrics 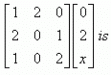
- a)
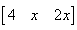
- b)
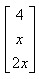
- c)
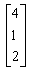
- d)
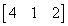
Correct answer is option 'B'. Can you explain this answer?
The product of two matrics 
a)
b)
c)
d)

|
Sushil Kumar answered |
{(1*0, 2*2, 0*x) (2*0, 0*2, 1*x) (1*0, 0*2, 2*x)}
= {4, x, 2x}
= {4, x, 2x}
If A and B are two matrices conformable to multiplication such that their product AB = O(Zero matrix). Then which of the following can be true
- a)A and B are both null matrices
- b)Either of A is or B is a null matrix
- c)Either of them may be a zero matrix
- d)It is Not necessary that A = 0 or B = 0
Correct answer is option 'D'. Can you explain this answer?
If A and B are two matrices conformable to multiplication such that their product AB = O(Zero matrix). Then which of the following can be true
a)
A and B are both null matrices
b)
Either of A is or B is a null matrix
c)
Either of them may be a zero matrix
d)
It is Not necessary that A = 0 or B = 0
|
|
Gaurav Kumar answered |
AB = 0 does not necessarily imply that either A or B is a null matrix
- Both matrices need not be null matrices.
- Both matrices need not be null matrices.
For the cell, 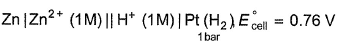 and for the cell Pt(H2) | H+ (1M)| Ag,
and for the cell Pt(H2) | H+ (1M)| Ag, 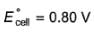 Thus Ecell for theAg|Ag+ (0.1M) || Zn2+ (0.1M) | Zn is ....................and cell reaction is...............
Thus Ecell for theAg|Ag+ (0.1M) || Zn2+ (0.1M) | Zn is ....................and cell reaction is...............- a)1.44 V ,spontaneous
- b)0.4 V ,spontaneous
- c)-1.44 V ,non-spontaneous
- d)-1.53 V ,non-spontaneous
Correct answer is option 'D'. Can you explain this answer?
For the cell, 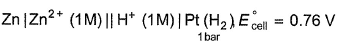 and for the cell Pt(H2) | H+ (1M)| Ag,
and for the cell Pt(H2) | H+ (1M)| Ag, 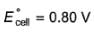
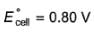
Thus Ecell for the
Ag|Ag+ (0.1M) || Zn2+ (0.1M) | Zn is ....................and cell reaction is...............
a)
1.44 V ,spontaneous
b)
0.4 V ,spontaneous
c)
-1.44 V ,non-spontaneous
d)
-1.53 V ,non-spontaneous
|
|
Geetika Shah answered |
Ecell < 0, hence reaction is non-spontaneous.
If A is a matrix of order 1×3 and B is a matrix of order 3×4, then order of the matrix obtained on multiplying A and B is- a)3×3
- b)4×1
- c)3×4
- d)1×4
Correct answer is option 'D'. Can you explain this answer?
If A is a matrix of order 1×3 and B is a matrix of order 3×4, then order of the matrix obtained on multiplying A and B is
a)
3×3
b)
4×1
c)
3×4
d)
1×4
|
|
Nandini Iyer answered |
In matrix 1*3 is one row and 3 columns and in 3*4 is three rows and four column hence multiplied matrix will be 1*4.
For the following cell with hydrogen electrodes at two different pressure p1 and p2 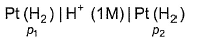 emf is given by
emf is given by- a)
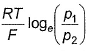
- b)
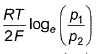
- c)
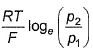
- d)
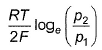
Correct answer is option 'B'. Can you explain this answer?
For the following cell with hydrogen electrodes at two different pressure p1 and p2 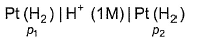
emf is given by
a)
b)
c)
d)
|
|
Krishna Iyer answered |
For SHE E°SHE = 0.00 V
Oxidation at anode (left)
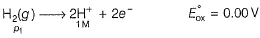
Reduction at cathode (right)
Net
Oxidation at anode (left)
Reduction at cathode (right)
Net
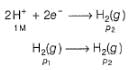
This is the type of the cell in which electrodes at different pressures are dipped in same electrolyte and connectivity is made by a salt-bridge.
Reaction Quotient (Q) 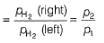
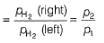
∵
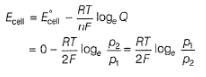
Electric potential due to a point charge q at a distance r from the point is _______ (in the air).- a)q/r
- b)q*r
- c)q/r2
- d)-q/r
Correct answer is option 'A'. Can you explain this answer?
Electric potential due to a point charge q at a distance r from the point is _______ (in the air).
a)
q/r
b)
q*r
c)
q/r2
d)
-q/r
|
|
Om Desai answered |
Force on a unit point charge kept at a distance r from the charge = q/r2.
Therefore, work done to bring that point charge through a small distance dr = q/r2 * (-dr). Therefore, the potential of that point is = 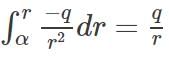 .
.
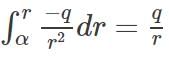 .
.A solution of Fe2+ is titrated potentiometrically using Ce4+ solution.Fe2+ → Fe3+ + e- , E0 = -0.77 Vemf of the Pt | Fe2+ , Fe3+ pair at 50% and 90% titration of Fe2+ are - a)0.77 V , 0.826 V
- b)-0.826 V , -0.77 V
- c)-0.77 V ,-0.826 V
- d)0.00 V , 0.00 V
Correct answer is option 'C'. Can you explain this answer?
A solution of Fe2+ is titrated potentiometrically using Ce4+ solution.
Fe2+ → Fe3+ + e- , E0 = -0.77 V
emf of the Pt | Fe2+ , Fe3+ pair at 50% and 90% titration of Fe2+ are
a)
0.77 V , 0.826 V
b)
-0.826 V , -0.77 V
c)
-0.77 V ,-0.826 V
d)
0.00 V , 0.00 V
|
|
Neha Chauhan answered |
Is oxidized to Fe3 by Ce4. The balanced chemical equation for the reaction is:
Fe2+ + Ce4+ → Fe3+ + Ce3+
The endpoint of the titration is reached when all the Fe2+ has been oxidized to Fe3+. This is indicated by a sudden increase in the potential of the solution. The potential of the Ce4+ solution is measured throughout the titration, and a plot of potential versus volume of Ce4+ added is used to determine the endpoint.
The standard potential of the Ce4+/Ce3+ couple is known, so the amount of Fe2+ in the original solution can be calculated from the volume and concentration of the Ce4+ solution used.
Potentiometric titration is a precise and accurate method for determining the concentration of Fe2+ in a solution. It is commonly used in analytical chemistry for the analysis of metal ions in solution.
Fe2+ + Ce4+ → Fe3+ + Ce3+
The endpoint of the titration is reached when all the Fe2+ has been oxidized to Fe3+. This is indicated by a sudden increase in the potential of the solution. The potential of the Ce4+ solution is measured throughout the titration, and a plot of potential versus volume of Ce4+ added is used to determine the endpoint.
The standard potential of the Ce4+/Ce3+ couple is known, so the amount of Fe2+ in the original solution can be calculated from the volume and concentration of the Ce4+ solution used.
Potentiometric titration is a precise and accurate method for determining the concentration of Fe2+ in a solution. It is commonly used in analytical chemistry for the analysis of metal ions in solution.
If 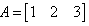 and
and 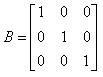 then AB = ?
then AB = ?
- a)[0]
- b)B
- c)
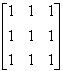
- d)A
Correct answer is option 'D'. Can you explain this answer?
If 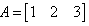 and
and 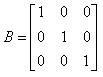 then AB = ?
then AB = ?
a)
[0]
b)
B
c)
d)
A

|
Tannya Tripathi answered |
By using multiplication of matrices, we know that no of columns of A should be equal to no of rows of B. so the order of matrix AB is1*1. here B is a identity matrix . and any matrix multiplied to identity matrix I is the matrix itself. hence AB=A.
A concentration cell reversible to anion (Cl-) is set up 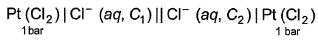 cell reaction is spontaneous ,if
cell reaction is spontaneous ,if - a)C1 > C2
- b)C1 < C2
- c)C1 = C2
- d)C1 = 0
Correct answer is option 'A'. Can you explain this answer?
A concentration cell reversible to anion (Cl-) is set up
cell reaction is spontaneous ,if
a)
C1 > C2
b)
C1 < C2
c)
C1 = C2
d)
C1 = 0
|
|
Om Desai answered |
This is a type of concentration cell with gas electrodes at the same pressure (1 bar) but dipped in aqueous solution of different concentration. Hence, a potential difference is set up.
At anode
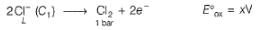
At cathode
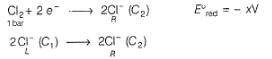
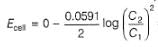
= 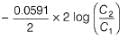
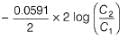
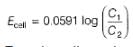
To make cell reaction spontaneous, Ecell > 0, hence C, > C2
Value of determinant is computed by adding multiples of one row to- a)another dimension
- b)another row
- c)another column
- d)another matrix
Correct answer is option 'B'. Can you explain this answer?
Value of determinant is computed by adding multiples of one row to
a)
another dimension
b)
another row
c)
another column
d)
another matrix
|
|
Lalit Yadav answered |
Value of Determinant remains unchanged if we add equal multiples of all the elements of row (column) to corresponding elements of another row (column) If, we have a given matrix A.
If 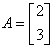 and
and 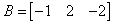 , then AB = ?
, then AB = ?- a)
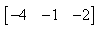
- b)
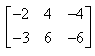
- c)
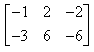
- d)
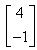
Correct answer is option 'B'. Can you explain this answer?
If 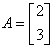 and
and 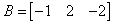 , then AB = ?
, then AB = ?
a)
b)
c)
d)

|
Tanoy Rudra answered |
Always check out for the new order and compatability of the order of both the matrices A & B. Then, multiply first Row element of A i.e. 2 with B's first column i.e [-1 2 -2] and then 2nd row element of A i.e 3 with [-1 2 -2].Resulting order will be of {2x 1(A) & 1x 3(B) } is 2 x 3.Hence , the given option B.
If A, B are, respectively m × n, k × l matrices, then both AB and BA are defined if and only if - a)n = m , k = m
- b)m = l , n= l
- c)m = n, k = l
- d)n = k and l = m.
Correct answer is option 'D'. Can you explain this answer?
If A, B are, respectively m × n, k × l matrices, then both AB and BA are defined if and only if
a)
n = m , k = m
b)
m = l , n= l
c)
m = n, k = l
d)
n = k and l = m.
|
|
Nikita Singh answered |
If A, B are, respectively m × n, k × l matrices, then both AB and BA are defined if and only if n = k and l = m. In particular, if both A and B are square matrices of the same order, then both AB and BA are defined.
Calculate electric potential due to a point charge of 10C at a distance of 8cm away from the charge.
- a)1.125*1013V
- b)1.125*1012V
- c)2.25*1013V
- d)0.62*1013V
Correct answer is option 'B'. Can you explain this answer?
Calculate electric potential due to a point charge of 10C at a distance of 8cm away from the charge.
a)
1.125*1013V
b)
1.125*1012V
c)
2.25*1013V
d)
0.62*1013V
|
|
Nikita Singh answered |
In the SI system, electric potential due to a point charge at a distance r is 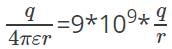 .
.
Substituting the values, we get potential =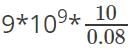
V = 1.125*1012V. Though in practice, this huge value of electric potential is not present.
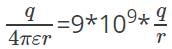 .
.Substituting the values, we get potential =
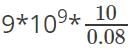
V = 1.125*1012V. Though in practice, this huge value of electric potential is not present.
Electric field intensity and electric potential at a certain distance from a point charge is 32 N/C and 16 J/C. What is the distance from the charge?- a)50 m
- b)0.5 m
- c)10 m
- d)7 m
Correct answer is option 'B'. Can you explain this answer?
Electric field intensity and electric potential at a certain distance from a point charge is 32 N/C and 16 J/C. What is the distance from the charge?
a)
50 m
b)
0.5 m
c)
10 m
d)
7 m
|
|
Nikita Singh answered |
Electric field due to a charge q at a distance r is 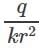 and electric potential at that point is
and electric potential at that point is 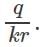 Therefore,
Therefore, 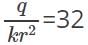 and
and 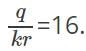 Dividing these two equations, we get r=0.5m. If the medium is air, k=1. Thus we can get the value of q=0.89 C.
Dividing these two equations, we get r=0.5m. If the medium is air, k=1. Thus we can get the value of q=0.89 C.
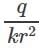 and electric potential at that point is
and electric potential at that point is 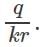 Therefore,
Therefore, 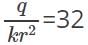 and
and 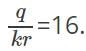 Dividing these two equations, we get r=0.5m. If the medium is air, k=1. Thus we can get the value of q=0.89 C.
Dividing these two equations, we get r=0.5m. If the medium is air, k=1. Thus we can get the value of q=0.89 C.Two half-cells are given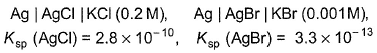 For a spontaneous cell reaction, cell set up is
For a spontaneous cell reaction, cell set up is - a)Ag| AgCI| KCI (0.2M) 11 KBr (0.001 M)| AgBr| Ag
- b)Ag| AgBr| KBr (0.001 M) 11 KCI (0.2 M) | AgCI | Ag
- c)Both (a) and (b)
- d)None of the above
Correct answer is option 'A,B'. Can you explain this answer?
Two half-cells are given
For a spontaneous cell reaction, cell set up is
a)
Ag| AgCI| KCI (0.2M) 11 KBr (0.001 M)| AgBr| Ag
b)
Ag| AgBr| KBr (0.001 M) 11 KCI (0.2 M) | AgCI | Ag
c)
Both (a) and (b)
d)
None of the above
|
|
Riya Banerjee answered |
Ksp (AgCI) = 2.8 x 10-10
[Ag+] [Cl-] = 2 .8 x 10-10
[Ag+] [Cl-] = 2 .8 x 10-10
∴ [Ag+]left = 
Ksp (AgBr) = 3.3 x 10-12
[Ag+][Br-] = 3.3 x 10-13

Ksp (AgBr) = 3.3 x 10-12
[Ag+][Br-] = 3.3 x 10-13
∴ [Ag+]left = 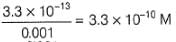
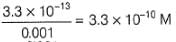
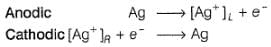
Net
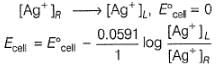
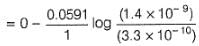
= -0.037 V
Thus, cell reaction is non-spontaneous
(b) Cell is reversed of (a), thus spontaneous.
Thus, cell reaction is non-spontaneous
(b) Cell is reversed of (a), thus spontaneous.
Three positive charges are kept at the vertices of an equilateral triangle. We can make the potential energy of the system zero by adjusting the amount of charges. This statement is ______- a)True
- b)False
Correct answer is option 'B'. Can you explain this answer?
Three positive charges are kept at the vertices of an equilateral triangle. We can make the potential energy of the system zero by adjusting the amount of charges. This statement is ______
a)
True
b)
False
|
|
Shalini Patel answered |
Electric potential is a positive quantity if both the charges are positive. In this case, all the three charges are positive; hence there is no negative term in the total energy term. Therefore, we cannot make the total energy of the system zero by adjusting the values of charge. But it would be possible if one of the charges were positive.
One or More than One Options Correct TypeThis section contains 4 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).- a)If salt-bridge is removed,potentials falls to zero
- b)Quinhydrone electrode is reversible to H+ ion
- c)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are used
- d)Calomel electrode contains calcium chloride solution in contact with Pt electrode
Correct answer is option 'A,B,C'. Can you explain this answer?
One or More than One Options Correct Type
This section contains 4 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Select the correct statement(s).
a)
If salt-bridge is removed,potentials falls to zero
b)
Quinhydrone electrode is reversible to H+ ion
c)
Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are used
d)
Calomel electrode contains calcium chloride solution in contact with Pt electrode
|
|
Raghavendra Rane answered |
a) If salt-bridge is removed, anodic and cathodic solution intermix and potential difference falls to zero — correct.
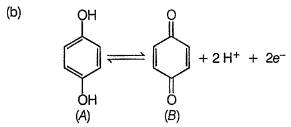
Quinhydrone is a mixture of A and S in (1:1) molar ratio joined by H-bonding. Above equilibrium is set up in aqueous solution.
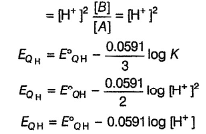
If [H+] changes, EQH also changes. Thus, quinhydrone electrode is reversible to H+ ion correct
(c) Liquid junction potential is minimised by use of concentration cell— correct.
(d) Calomel electrode is Hg(/), Hg2Cl2 (s)| Cl-.
Thus, (d) is incorrect.
Quinhydrone is a mixture of A and S in (1:1) molar ratio joined by H-bonding. Above equilibrium is set up in aqueous solution.
If [H+] changes, EQH also changes. Thus, quinhydrone electrode is reversible to H+ ion correct
(c) Liquid junction potential is minimised by use of concentration cell— correct.
(d) Calomel electrode is Hg(/), Hg2Cl2 (s)| Cl-.
Thus, (d) is incorrect.
Only One Option Correct TypeThis section contains 18 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Which cell will measure the standard electrode potential of zinc electrode?- a)P t(s)|H2(g, 0.1 bar) | H+ (aq, 0.1 M) || Zn2+ (aq, 0.1 M)|
- b)P t(s)|H2(g, 0.1 bar) | H+ (aq, 0.1 M) || Zn2+ (aq, 0.2 M)| Zn
- c)P t(s)|H2(g, 0.1 bar) | H+ (aq, 0.1 M) || Zn2+ (aq, 1.0 M)| Zn
- d)P t(s)|H2(g, 0.1 bar) | H+ (aq, 1.0 M) || Zn2+ (aq, 0.1 M)| Zn
Correct answer is option 'C'. Can you explain this answer?
Only One Option Correct Type
This section contains 18 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which cell will measure the standard electrode potential of zinc electrode?
a)
P t(s)|H2(g, 0.1 bar) | H+ (aq, 0.1 M) || Zn2+ (aq, 0.1 M)|
b)
P t(s)|H2(g, 0.1 bar) | H+ (aq, 0.1 M) || Zn2+ (aq, 0.2 M)| Zn
c)
P t(s)|H2(g, 0.1 bar) | H+ (aq, 0.1 M) || Zn2+ (aq, 1.0 M)| Zn
d)
P t(s)|H2(g, 0.1 bar) | H+ (aq, 1.0 M) || Zn2+ (aq, 0.1 M)| Zn

|
Mrinalini Chopra answered |
Standard electrode potential is the potential difference under standard state, i.e.
When [Mn+] = 1M
It can be measured by coupling it with SHE electrode
Pt (s) | H2(gr. 1 bar) | H+ (1.0 M)
When [Mn+] = 1M
It can be measured by coupling it with SHE electrode
Pt (s) | H2(gr. 1 bar) | H+ (1.0 M)
Three charges –q, Q and –q are placed in a straight line maintaining equal distance from each other. What should be the ratio q/Q so that the net electric potential of the system is zero?- a)1
- b)2
- c)3
- d)4
Correct answer is option 'D'. Can you explain this answer?
Three charges –q, Q and –q are placed in a straight line maintaining equal distance from each other. What should be the ratio q/Q so that the net electric potential of the system is zero?
a)
1
b)
2
c)
3
d)
4
|
|
Rajesh Chauhan answered |
Assuming you are asking about three point charges in space:
Three point charges are placed in space as follows: q1 = +2.0 μC, q2 = -3.0 μC, and q3 = +4.0 μC. The charges are located at the vertices of an equilateral triangle with sides of length 0.50 m.
a) What is the electric potential energy of the system?
b) What is the magnitude and direction of the net force on q1?
c) At what point on the x-axis (other than infinity) is the electric potential zero?
Solution:
a) The electric potential energy of a system of point charges is given by the formula:
U = k * (q1 * q2 / r12 + q1 * q3 / r13 + q2 * q3 / r23)
where k is the Coulomb constant, q1, q2, and q3 are the charges, and r12, r13, and r23 are the distances between them. In this case, the charges are located at the vertices of an equilateral triangle with sides of length 0.50 m. The distances between them are all equal and can be calculated using the Pythagorean theorem:
r12 = r13 = r23 = 0.50 m
Substituting the values into the formula, we get:
U = 9.0 * 10^9 * [(2 * 10^-6 * (-3 * 10^-6)) / 0.50 + (2 * 10^-6 * 4 * 10^-6) / 0.50 + (-3 * 10^-6 * 4 * 10^-6) / 0.50]
U = -3.96 * 10^-5 J
Therefore, the electric potential energy of the system is -3.96 * 10^-5 J.
b) The net force on q1 is the vector sum of the forces due to q2 and q3. The force between two point charges is given by Coulomb's law:
F12 = k * (q1 * q2 / r12^2) * u12
where u12 is the unit vector pointing from q1 to q2. Similarly, the force between q1 and q3 is given by:
F13 = k * (q1 * q3 / r13^2) * u13
where u13 is the unit vector pointing from q1 to q3. The magnitude and direction of these forces can be calculated using the formula:
|F| = k * (|q1| * |q2| / r^2)
where r is the distance between the charges and |q| is the absolute value of the charge. The unit vectors can be calculated using the formula:
u = (r2 - r1) / |r2 - r1|
where r1 and r2 are the position vectors of the charges.
Substituting the values, we get:
F12 = 2.07 * 10^-3 N, u12 = (-i + √3 * j) / 2
F13 = 3.10 * 10^-3 N, u13 = (i + √3 * j) / 2
The net force on q1 is the vector sum of F12 and F13:
Three point charges are placed in space as follows: q1 = +2.0 μC, q2 = -3.0 μC, and q3 = +4.0 μC. The charges are located at the vertices of an equilateral triangle with sides of length 0.50 m.
a) What is the electric potential energy of the system?
b) What is the magnitude and direction of the net force on q1?
c) At what point on the x-axis (other than infinity) is the electric potential zero?
Solution:
a) The electric potential energy of a system of point charges is given by the formula:
U = k * (q1 * q2 / r12 + q1 * q3 / r13 + q2 * q3 / r23)
where k is the Coulomb constant, q1, q2, and q3 are the charges, and r12, r13, and r23 are the distances between them. In this case, the charges are located at the vertices of an equilateral triangle with sides of length 0.50 m. The distances between them are all equal and can be calculated using the Pythagorean theorem:
r12 = r13 = r23 = 0.50 m
Substituting the values into the formula, we get:
U = 9.0 * 10^9 * [(2 * 10^-6 * (-3 * 10^-6)) / 0.50 + (2 * 10^-6 * 4 * 10^-6) / 0.50 + (-3 * 10^-6 * 4 * 10^-6) / 0.50]
U = -3.96 * 10^-5 J
Therefore, the electric potential energy of the system is -3.96 * 10^-5 J.
b) The net force on q1 is the vector sum of the forces due to q2 and q3. The force between two point charges is given by Coulomb's law:
F12 = k * (q1 * q2 / r12^2) * u12
where u12 is the unit vector pointing from q1 to q2. Similarly, the force between q1 and q3 is given by:
F13 = k * (q1 * q3 / r13^2) * u13
where u13 is the unit vector pointing from q1 to q3. The magnitude and direction of these forces can be calculated using the formula:
|F| = k * (|q1| * |q2| / r^2)
where r is the distance between the charges and |q| is the absolute value of the charge. The unit vectors can be calculated using the formula:
u = (r2 - r1) / |r2 - r1|
where r1 and r2 are the position vectors of the charges.
Substituting the values, we get:
F12 = 2.07 * 10^-3 N, u12 = (-i + √3 * j) / 2
F13 = 3.10 * 10^-3 N, u13 = (i + √3 * j) / 2
The net force on q1 is the vector sum of F12 and F13:
Electrode potential of the following half-cell is dependent on
Hg, HgO |OH-(aq)- a)temperature
- b)pH
- c)Both (a) and (b)
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Electrode potential of the following half-cell is dependent on
Hg, HgO |OH-(aq)
Hg, HgO |OH-(aq)
a)
temperature
b)
pH
c)
Both (a) and (b)
d)
None of these
|
|
Jhanvi Sengupta answered |
Explanation:
Temperature:
The electrode potential of a half-cell is dependent on temperature. As the temperature increases, the rate of reaction at the electrode also increases, leading to a change in the electrode potential. In the case of the Hg, HgO |OH-(aq) half-cell, the temperature can affect the reaction between mercury and mercury(II) oxide, thus impacting the electrode potential.
pH:
The pH of the solution in the half-cell also plays a crucial role in determining the electrode potential. The concentration of hydroxide ions (OH-) in the solution can affect the reaction at the electrode and, consequently, the electrode potential. A change in pH can alter the distribution of species in the solution, leading to a change in the electrode potential.
Both (a) and (b):
In the given half-cell (Hg, HgO |OH-(aq)), both temperature and pH can influence the electrode potential. Therefore, the correct answer is option C, which states that the electrode potential is dependent on both temperature and pH in this specific half-cell setup.
In conclusion, the electrode potential of the Hg, HgO |OH-(aq) half-cell is influenced by both temperature and pH. It is essential to consider these factors when studying the electrochemistry of this particular half-cell.
Temperature:
The electrode potential of a half-cell is dependent on temperature. As the temperature increases, the rate of reaction at the electrode also increases, leading to a change in the electrode potential. In the case of the Hg, HgO |OH-(aq) half-cell, the temperature can affect the reaction between mercury and mercury(II) oxide, thus impacting the electrode potential.
pH:
The pH of the solution in the half-cell also plays a crucial role in determining the electrode potential. The concentration of hydroxide ions (OH-) in the solution can affect the reaction at the electrode and, consequently, the electrode potential. A change in pH can alter the distribution of species in the solution, leading to a change in the electrode potential.
Both (a) and (b):
In the given half-cell (Hg, HgO |OH-(aq)), both temperature and pH can influence the electrode potential. Therefore, the correct answer is option C, which states that the electrode potential is dependent on both temperature and pH in this specific half-cell setup.
In conclusion, the electrode potential of the Hg, HgO |OH-(aq) half-cell is influenced by both temperature and pH. It is essential to consider these factors when studying the electrochemistry of this particular half-cell.
Two point charges 10C and -10C are placed at a certain distance. What is the electric potential of their midpoint?- a)Some positive value
- b)Some negative value
- c)Zero
- d)Depends on medium
Correct answer is option 'C'. Can you explain this answer?
Two point charges 10C and -10C are placed at a certain distance. What is the electric potential of their midpoint?
a)
Some positive value
b)
Some negative value
c)
Zero
d)
Depends on medium
|
|
Anagha Mukherjee answered |
Electric Potential at the Midpoint of Two Point Charges
To find the electric potential at the midpoint of two point charges, we can use the principle of superposition. The electric potential at a point due to multiple charges is the algebraic sum of the electric potentials due to each individual charge.
Calculating Electric Potential
The electric potential at a point due to a point charge is given by the equation:
V = k * q / r
Where:
- V is the electric potential
- k is the electrostatic constant (9 x 10^9 Nm^2/C^2)
- q is the charge
- r is the distance between the charge and the point where the potential is being calculated
In this scenario, we have two point charges: +10C and -10C. Let's assume the distance between them is 'd'. The midpoint is equidistant from both charges, so the distance from each charge to the midpoint is d/2.
Using the equation for electric potential, we can calculate the potential at the midpoint due to each charge separately:
V1 = k * (+10C) / (d/2)
V2 = k * (-10C) / (d/2)
Since the charges have equal magnitudes but opposite signs, their potentials will have equal magnitudes but opposite signs as well.
Applying Superposition Principle
To find the electric potential at the midpoint, we need to add the potentials due to each charge:
V_total = V1 + V2
Considering their magnitudes and opposite signs, we have:
V_total = (k * (+10C) / (d/2)) + (k * (-10C) / (d/2))
= (10C * k / (d/2)) - (10C * k / (d/2))
= 0
Conclusion
Therefore, the electric potential at the midpoint of two point charges of +10C and -10C is zero. This means that the potential at the midpoint is the same as if there were no charges present. The cancellation of potentials is a result of the equal but opposite charges and the symmetry of the arrangement.
Hence, the correct answer is option c) Zero.
To find the electric potential at the midpoint of two point charges, we can use the principle of superposition. The electric potential at a point due to multiple charges is the algebraic sum of the electric potentials due to each individual charge.
Calculating Electric Potential
The electric potential at a point due to a point charge is given by the equation:
V = k * q / r
Where:
- V is the electric potential
- k is the electrostatic constant (9 x 10^9 Nm^2/C^2)
- q is the charge
- r is the distance between the charge and the point where the potential is being calculated
In this scenario, we have two point charges: +10C and -10C. Let's assume the distance between them is 'd'. The midpoint is equidistant from both charges, so the distance from each charge to the midpoint is d/2.
Using the equation for electric potential, we can calculate the potential at the midpoint due to each charge separately:
V1 = k * (+10C) / (d/2)
V2 = k * (-10C) / (d/2)
Since the charges have equal magnitudes but opposite signs, their potentials will have equal magnitudes but opposite signs as well.
Applying Superposition Principle
To find the electric potential at the midpoint, we need to add the potentials due to each charge:
V_total = V1 + V2
Considering their magnitudes and opposite signs, we have:
V_total = (k * (+10C) / (d/2)) + (k * (-10C) / (d/2))
= (10C * k / (d/2)) - (10C * k / (d/2))
= 0
Conclusion
Therefore, the electric potential at the midpoint of two point charges of +10C and -10C is zero. This means that the potential at the midpoint is the same as if there were no charges present. The cancellation of potentials is a result of the equal but opposite charges and the symmetry of the arrangement.
Hence, the correct answer is option c) Zero.
Which has the maximum potential at 298 K (numerical value) for the half-cell reaction?2H+ + 2e- → H2 (1 bar) - a)1.0 M HCl
- b)A solution having pH 4
- c)Pure water
- d)1.0 M NaOH
Correct answer is option 'D'. Can you explain this answer?
Which has the maximum potential at 298 K (numerical value) for the half-cell reaction?
2H+ + 2e- → H2 (1 bar)
a)
1.0 M HCl
b)
A solution having pH 4
c)
Pure water
d)
1.0 M NaOH

|
Nisha Banerjee answered |
2H+ + 2e- → H2
This represents reduction half-cell reaction
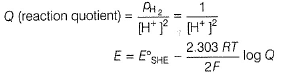
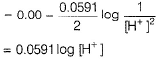
(a) [H+] = 1 M, E = 0
(b) pH = 4, [H+] = 10-4M
∴ E = 0.0591 log 10-4
= - 4 x 0.0591 = - 0.2364 V
(c) Pure water, [H+] = 10-7M
∴ E = 0.0591 log 10-7
= - 7 x 0,0591 = - 0.4137 V
(d) 1.0 M NaOH
[OH-] = 1 M
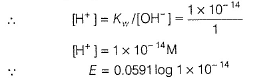
∴ E = 0.0591 log 1 x 10-14
= -14 x 0.0591
= - 0.8274 V (maximum)
This represents reduction half-cell reaction
(a) [H+] = 1 M, E = 0
(b) pH = 4, [H+] = 10-4M
∴ E = 0.0591 log 10-4
= - 4 x 0.0591 = - 0.2364 V
(c) Pure water, [H+] = 10-7M
∴ E = 0.0591 log 10-7
= - 7 x 0,0591 = - 0.4137 V
(d) 1.0 M NaOH
[OH-] = 1 M
∴ E = 0.0591 log 1 x 10-14
= -14 x 0.0591
= - 0.8274 V (maximum)
Following half-cell, Pt (H2)|H2O behaves as SHE at a pressure of- a)1 bar
- b)10-14 bar
- c)107 bar
- d)1014 bar
Correct answer is option 'B'. Can you explain this answer?
Following half-cell, Pt (H2)|H2O behaves as SHE at a pressure of
a)
1 bar
b)
10-14 bar
c)
107 bar
d)
1014 bar

|
Ishita Deshpande answered |
Pt(H2)| H2O
This is oxidation half-cell. Instead of
[H+] = 1 M and pH2 = 1 bar
H2O has been taken as a source of [H+]
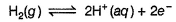
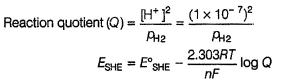
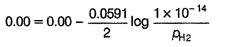
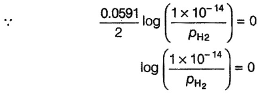
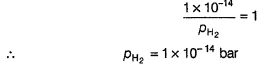
This is oxidation half-cell. Instead of
[H+] = 1 M and pH2 = 1 bar
H2O has been taken as a source of [H+]
What is the amount of work done to bring a charge of 4*10-3C charge from infinity to a point whose electric potential is 2*102V?- a)0.8J
- b)-0.8J
- c)1.6J
- d)-0.4J
Correct answer is option 'A'. Can you explain this answer?
What is the amount of work done to bring a charge of 4*10-3C charge from infinity to a point whose electric potential is 2*102V?
a)
0.8J
b)
-0.8J
c)
1.6J
d)
-0.4J
|
|
Rutuja Ahuja answered |
To find the amount of work done to bring a charge of 4*10^-3C from infinity to a point whose electric potential is 2*10^2V, we can use the equation:
Work done = q * ΔV
Where:
q is the charge (4*10^-3C)
ΔV is the change in electric potential (2*10^2V)
Let's calculate the work done step by step:
Step 1: Calculate the work done.
Work done = (4*10^-3C) * (2*10^2V)
Step 2: Simplify the expression.
Work done = 8*10^-1C * 10^2V
Step 3: Use the property of exponents.
Work done = 8 * 10^-1 * 10^2 * C * V
Step 4: Multiply the numbers.
Work done = 8 * 10^1 * C * V
Step 5: Simplify the expression.
Work done = 8 * 10 * C * V
Step 6: Multiply the numbers.
Work done = 80 * C * V
Step 7: Substitute the values of C and V.
Work done = 80 * 4*10^-3C * 2*10^2V
Step 8: Simplify the expression.
Work done = 80 * 4 * 10^-3 * 2 * 10^2 * C * V
Step 9: Multiply the numbers.
Work done = 80 * 4 * 2 * 10^-3 * 10^2 * C * V
Step 10: Simplify the expression.
Work done = 80 * 4 * 2 * 10^-3 * 10^2 * C * V
Work done = 320 * 10^-1 * C * V
Work done = 320 * 10^-1 * CV
Step 11: Substitute the values of C and V.
Work done = 320 * 10^-1 * 4*10^-3C * 2*10^2V
Work done = 320 * 10^-1 * 4 * 2 * 10^-3 * 10^2 * C * V
Work done = 320 * 4 * 2 * 10^-1 * 10^2 * C * V
Work done = 320 * 4 * 2 * 10^-1 * 10^2 * C * V
Work done = 320 * 8 * 10^-1 * 10^2 * C * V
Step 12: Simplify the expression.
Work done = 2560 * 10^-1 * C * V
Work done = 2560 * 10^-1 * CV
Step 13: Substitute the values of C and V.
Work done = 2560 * 10^-1 * 4*10^-3C * 2*10^2V
Work done = 2560 * 10^-1 * 4 * 2 * 10^-3 * 10^2 * C * V
Work done = 2560 * 4 * 2 * 10^-1 * 10^2 * C * V
Work done = 2560 * 4 *
Work done = q * ΔV
Where:
q is the charge (4*10^-3C)
ΔV is the change in electric potential (2*10^2V)
Let's calculate the work done step by step:
Step 1: Calculate the work done.
Work done = (4*10^-3C) * (2*10^2V)
Step 2: Simplify the expression.
Work done = 8*10^-1C * 10^2V
Step 3: Use the property of exponents.
Work done = 8 * 10^-1 * 10^2 * C * V
Step 4: Multiply the numbers.
Work done = 8 * 10^1 * C * V
Step 5: Simplify the expression.
Work done = 8 * 10 * C * V
Step 6: Multiply the numbers.
Work done = 80 * C * V
Step 7: Substitute the values of C and V.
Work done = 80 * 4*10^-3C * 2*10^2V
Step 8: Simplify the expression.
Work done = 80 * 4 * 10^-3 * 2 * 10^2 * C * V
Step 9: Multiply the numbers.
Work done = 80 * 4 * 2 * 10^-3 * 10^2 * C * V
Step 10: Simplify the expression.
Work done = 80 * 4 * 2 * 10^-3 * 10^2 * C * V
Work done = 320 * 10^-1 * C * V
Work done = 320 * 10^-1 * CV
Step 11: Substitute the values of C and V.
Work done = 320 * 10^-1 * 4*10^-3C * 2*10^2V
Work done = 320 * 10^-1 * 4 * 2 * 10^-3 * 10^2 * C * V
Work done = 320 * 4 * 2 * 10^-1 * 10^2 * C * V
Work done = 320 * 4 * 2 * 10^-1 * 10^2 * C * V
Work done = 320 * 8 * 10^-1 * 10^2 * C * V
Step 12: Simplify the expression.
Work done = 2560 * 10^-1 * C * V
Work done = 2560 * 10^-1 * CV
Step 13: Substitute the values of C and V.
Work done = 2560 * 10^-1 * 4*10^-3C * 2*10^2V
Work done = 2560 * 10^-1 * 4 * 2 * 10^-3 * 10^2 * C * V
Work done = 2560 * 4 * 2 * 10^-1 * 10^2 * C * V
Work done = 2560 * 4 *
Two plates are kept at a distance of 0.1m and their potential difference is 20V. An electron is kept at rest on the surface of the plate with lower potential. What will be the velocity of the electron when it strikes another plate?- a)1.87*106 m/s
- b)2.65*106 m/s
- c)7.02*1012 m/s
- d)32*10-19 m/s
Correct answer is option 'B'. Can you explain this answer?
Two plates are kept at a distance of 0.1m and their potential difference is 20V. An electron is kept at rest on the surface of the plate with lower potential. What will be the velocity of the electron when it strikes another plate?
a)
1.87*106 m/s
b)
2.65*106 m/s
c)
7.02*1012 m/s
d)
32*10-19 m/s
|
|
Aravind Rane answered |
Given data:
Distance between two plates (d) = 0.1m
Potential difference between two plates (V) = 20V
To find: Velocity of electron when it strikes another plate
Formula used:
The potential difference between two plates is given by: V = Ed
where E is the electric field between the plates and d is the distance between the plates.
The electron placed at the lower potential plate has potential energy (PE) given by:
PE = qV
where q is the charge of the electron and V is the potential difference between two plates.
The potential energy of the electron is converted into kinetic energy when the electron moves towards the higher potential plate. The kinetic energy of the electron is given by:
KE = 1/2mv^2
where m is the mass of the electron and v is the velocity of the electron.
As per conservation of energy, the potential energy of the electron is converted into kinetic energy. Therefore,
PE = KE
qV = 1/2mv^2
Solving for v, we get:
v = sqrt(2qV/m)
Substituting the given values, we get:
v = sqrt(2*1.6*10^-19*20/9.1*10^-31)
v = 2.65*10^6 m/s
Therefore, the velocity of the electron when it strikes another plate is 2.65*10^6 m/s.
Answer: Option B.
Distance between two plates (d) = 0.1m
Potential difference between two plates (V) = 20V
To find: Velocity of electron when it strikes another plate
Formula used:
The potential difference between two plates is given by: V = Ed
where E is the electric field between the plates and d is the distance between the plates.
The electron placed at the lower potential plate has potential energy (PE) given by:
PE = qV
where q is the charge of the electron and V is the potential difference between two plates.
The potential energy of the electron is converted into kinetic energy when the electron moves towards the higher potential plate. The kinetic energy of the electron is given by:
KE = 1/2mv^2
where m is the mass of the electron and v is the velocity of the electron.
As per conservation of energy, the potential energy of the electron is converted into kinetic energy. Therefore,
PE = KE
qV = 1/2mv^2
Solving for v, we get:
v = sqrt(2qV/m)
Substituting the given values, we get:
v = sqrt(2*1.6*10^-19*20/9.1*10^-31)
v = 2.65*10^6 m/s
Therefore, the velocity of the electron when it strikes another plate is 2.65*10^6 m/s.
Answer: Option B.
Comprehension TypeThis section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecell of Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Pure [Ag+] in the ore is - a)8.4 x 10-6 M
- b)2.9 x 10-6 M
- c)7.0 x 10-11 M
- d)4.2 x 10-6 M
Correct answer is option 'A'. Can you explain this answer?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage I
1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecell of Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 V
Q.
Pure [Ag+] in the ore is
a)
8.4 x 10-6 M
b)
2.9 x 10-6 M
c)
7.0 x 10-11 M
d)
4.2 x 10-6 M
|
|
Nandita Ahuja answered |
Cell Reaction
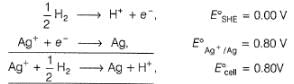
∴ x = 8.4 x 10-6 M
[Ag+] = 8.4 x 10-6 mol L-1
= 8.4 x 10-6 x 0.350 mol in 350 mL
= 8.4 x 10-6 x 0.350 x 108 g in 350 mL
= 3.1752 x 10-4 g in 1.05 g sample
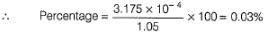
Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecell of Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is - a)0.03 %
- b)0.06 %
- c)3.4 %
- d)1.20 %
Correct answer is option 'A'. Can you explain this answer?
Passage I
1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecell of Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 V
Q.
Percentage of silver in the sample is
a)
0.03 %
b)
0.06 %
c)
3.4 %
d)
1.20 %

|
Aravind Mehra answered |
Method to Solve :
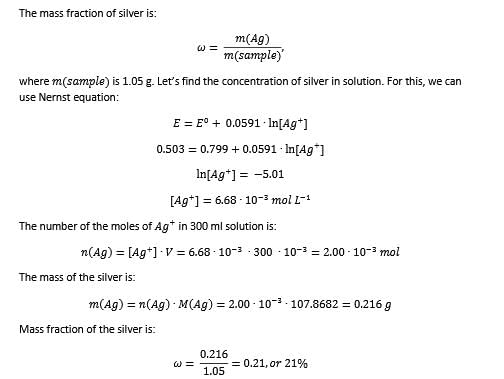
One Integer Value Correct TypeThis section contains 3 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)Q. For the following cell with metal X electrodes, 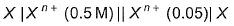 Ecell = -0.028 V at 298 K, if there is no liquid juncton potential,valency of X is.......
Ecell = -0.028 V at 298 K, if there is no liquid juncton potential,valency of X is.......
Correct answer is '2'. Can you explain this answer?
One Integer Value Correct Type
This section contains 3 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q.
For the following cell with metal X electrodes, 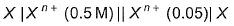
Ecell = -0.028 V at 298 K, if there is no liquid juncton potential,valency of X is.......

|
Srishti Kaur answered |
A small charge q is rotated in a complete circular path of radius r surrounding another charge Q. The work done in this process is _________- a)Zero
- b)
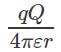
- c)
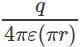
- d)
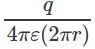
Correct answer is option 'A'. Can you explain this answer?
A small charge q is rotated in a complete circular path of radius r surrounding another charge Q. The work done in this process is _________
a)
Zero
b)
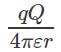
c)
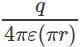
d)
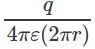
|
|
Shalini Patel answered |
We know that electric potential does not depend on the path, i.e. it is a state function. Therefore if we rotate a charge around another in a complete circular path, the potential energy of its initial and final points is the same. Therefore, there is no change of potential energy of the charge and hence net work done on the charge is zero.
In the following cell at 298 K,two weak acids (HA) and (HB) with pKa (HA) = 3 and pKa (HB) = 5 of equal molarity have been used as shown.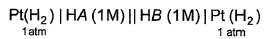 Thus, emf of the cell is
Thus, emf of the cell is - a)0.059 V
- b)-0.059 V
- c)
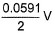
- d)
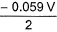
Correct answer is option 'B'. Can you explain this answer?
In the following cell at 298 K,two weak acids (HA) and (HB) with pKa (HA) = 3 and pKa (HB) = 5 of equal molarity have been used as shown.
Thus, emf of the cell is
a)
0.059 V
b)
-0.059 V
c)
d)

|
Nisha Banerjee answered |
Take HA as the right-hand electrode and HB as the left-hand electrode.
For Hydrogen electrode at 1 atm and 25�C
E red = -0.059pH
pH of weak acid = 1/2 pKa - 1/2 log C
so for the electrode HA E red = -0.059/2 [ pKa - log C] = - 0.0295 [3 - log C]
For electrode HB E red = -0.059/2 [ pKa - log C] = -0.0295 [5 - log C]
Ecell = E red of HA - E red of HB = - 0.0295 [3 - log C] + 0.0295 [5 - log C] = 0.059 V
Passage IIFor the following,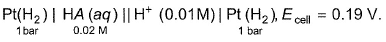 Q. pKa of the weak monobasic acid is
Q. pKa of the weak monobasic acid is - a)6.72
- b)4.50
- c)7.00
- d)8.72
Correct answer is option 'D'. Can you explain this answer?
Passage II
For the following,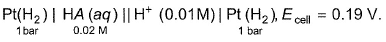
Q.
pKa of the weak monobasic acid is
a)
6.72
b)
4.50
c)
7.00
d)
8.72

|
Nisha Banerjee answered |
Anodic
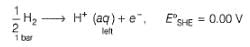
Cathodic
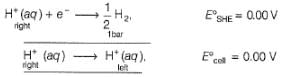
Reaction quotient (Q)
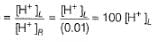

What is the electric potential of the system?
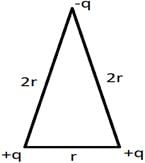
- a)
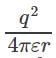
- b)

- c)Zero
- d)

Correct answer is option 'C'. Can you explain this answer?
What is the electric potential of the system?


a)
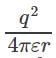
b)

c)
Zero
d)

|
|
Shalini Patel answered |
Electric potential between +q and +q is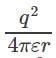 and electric potential between two charges +q and –q is
and electric potential between two charges +q and –q is 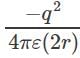 . Therefore the electric potential of the system is
. Therefore the electric potential of the system is  Electric potential is a scalar quantity and thus the system becomes zero-potential-system.
Electric potential is a scalar quantity and thus the system becomes zero-potential-system.
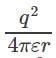 and electric potential between two charges +q and –q is
and electric potential between two charges +q and –q is 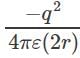 . Therefore the electric potential of the system is
. Therefore the electric potential of the system is  Electric potential is a scalar quantity and thus the system becomes zero-potential-system.
Electric potential is a scalar quantity and thus the system becomes zero-potential-system.Osmatic pressure of a 0.001 M weak monobasic acid (HA) at 300 K is 2.5 x 10-2 atm.Thus,emf of the follwing in decivolt is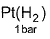 | 0.001 M HA........
| 0.001 M HA........
Correct answer is '3'. Can you explain this answer?
Osmatic pressure of a 0.001 M weak monobasic acid (HA) at 300 K is 2.5 x 10-2 atm.Thus,emf of the follwing in decivolt is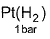 | 0.001 M HA........
| 0.001 M HA........

|
Maitri Sharma answered |
This question relates colligative properties to emf of the cell
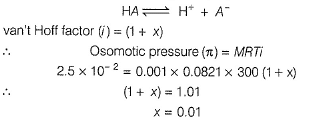
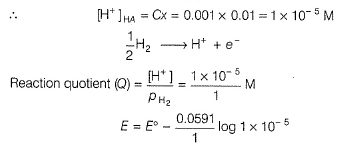

For the half-cell,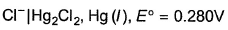 at 298K electrode potential has maximum value when KCl used is
at 298K electrode potential has maximum value when KCl used is - a)1 M
- b)0.1 M
- c)0.01 M
- d)10 M
Correct answer is option 'C'. Can you explain this answer?
For the half-cell,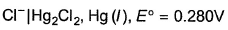 at 298K electrode potential has maximum value when KCl used is
at 298K electrode potential has maximum value when KCl used is
a)
1 M
b)
0.1 M
c)
0.01 M
d)
10 M
|
|
Anu Das answered |
Cl- / Hg2CI2, Hg (/)
This is reduction half-cell
Hg2CI2(s) + 2e- → 2Hg (/) + 2Cl-
Reaction quotient (Q) = [Cl-]2
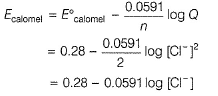
Thus, larger the value of [Cl-], smaller the value of Ecaiomel.
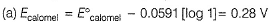
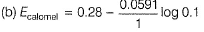
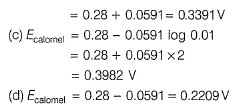
This is reduction half-cell
Hg2CI2(s) + 2e- → 2Hg (/) + 2Cl-
Reaction quotient (Q) = [Cl-]2
Thus, larger the value of [Cl-], smaller the value of Ecaiomel.
For Daniell cell E°cell = 1.10 V. If state of equilibrium is attained, then- a)
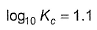
- b)
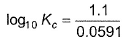
- c)
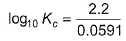
- d)
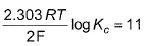
Correct answer is option 'C,D'. Can you explain this answer?
For Daniell cell E°cell = 1.10 V. If state of equilibrium is attained, then
a)
b)
c)
d)

|
Sravya Datta answered |
Reaction taking place in a Daniell cell is
Zn(s) + Cu2+ (1M)  Cu(s) + Zn2+ (1M),
Cu(s) + Zn2+ (1M), 
 Cu(s) + Zn2+ (1M),
Cu(s) + Zn2+ (1M), 
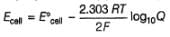
E°red (standard reduction electrode potentials) of different half-cell are given 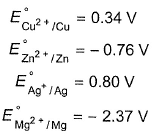 In which cell , is ΔG° most negative?
In which cell , is ΔG° most negative?- a)Zn|zn2+(1M)||Mg2+ (1M)|Mg
- b)Zn|zn2+ (1M) ||Ag+ (1M)|Ag
- c)Cu|Cu2+ (1M)||Ag+(1M) | Ag
- d)Ag|Ag+ (1M) || Mg2+ (1M)|Mg
Correct answer is option 'B'. Can you explain this answer?
E°red (standard reduction electrode potentials) of different half-cell are given
In which cell , is ΔG° most negative?
a)
Zn|zn2+(1M)||Mg2+ (1M)|Mg
b)
Zn|zn2+ (1M) ||Ag+ (1M)|Ag
c)
Cu|Cu2+ (1M)||Ag+(1M) | Ag
d)
Ag|Ag+ (1M) || Mg2+ (1M)|Mg

|
Ishita Deshpande answered |
In (b) E°cell is most positive, then ΔG° is most negative.
Chapter doubts & questions for May Week 4 - Weekly Tests for JEE Preparation 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of May Week 4 - Weekly Tests for JEE Preparation in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Related JEE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily